Long-term Effects of Mitiglinide in Japanese Diabetics Inadequately Controlled with DPP-4 Inhibitor or Biguanide Monotherapy
- PMID: 24488695
- PMCID: PMC4065298
- DOI: 10.1007/s13300-014-0051-5
Long-term Effects of Mitiglinide in Japanese Diabetics Inadequately Controlled with DPP-4 Inhibitor or Biguanide Monotherapy
Abstract
Introduction: The goal of treatment in diabetes is to control hyperglycemia to near-normal glucose levels, which is important to prevent the progression of microvascular and macrovascular complications. Mitiglinide is a rapid- and short-acting insulinotropic sulfonylurea receptor ligand that is known to improve postprandial hyperglycemia in patients with type 2 diabetes. The aim of this study was to investigate the long-term efficacy and safety of mitiglinide in Japanese type 2 diabetic patients inadequately controlled by dipeptidyl peptidase-4 (DPP-4) inhibitor or biguanide monotherapy.
Methods: In patients with type 2 diabetes mellitus (T2DM) receiving a stable monotherapy regimen with a DPP-4 inhibitor or biguanide added to dietary therapy, an additional 10 mg mitiglinide was administered for 52 weeks. The efficacy end points were postprandial plasma glucose (PPG) (30 min, 1 h, 2 h), postprandial insulin (30 min, 1 h, 2 h), insulinogenic index, 1,5-anhydroglucitol (1,5-AG), glycated hemoglobin (HbA1c), and fasting plasma glucose. The safety end points included the incidence and types of adverse events and adverse drug reactions.
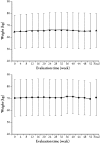
Results: A total of 136 patients with T2DM were eligible for enrollment in this study and received mitiglinide. The average HbA1c before the start of mitiglinide administration (baseline) was 7.47% in the DPP-4 inhibitor combined treatment group (DPP-4 inhibitor CTG) and 7.50% in the biguanide combined treatment group (biguanide CTG), and the 2 h PPG was 248.1 and 243.3 mg/dL, respectively. Following the addition of mitiglinide to the treatment regimen for 52 weeks, the early postprandial decrease in insulin secretion improved and PPG improved in both the DPP-4 inhibitor CTG and biguanide CTG. At final evaluation, the HbA1c <7.0% achievement rate was 57.4% in the DPP-4 inhibitor CTG and 29.2% in the biguanide CTG. The incidence of hypoglycemia in the DPP-4 inhibitor CTG and biguanide CTG was 3.0% (2/67 patients) and 2.9% (2/69 patients), respectively. The hypoglycemic symptoms were mild in all cases.
Conclusion: Combination therapy with mitiglinide and DPP-4 inhibitors or biguanides improved glycemic control over the long term without increasing risks to safety due to events such as hypoglycemia, and this is a clinically promising therapeutic strategy in T2DM.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

