Two-component histidine phosphotransfer protein Ypd1 is not essential for viability in Candida albicans
- PMID: 24489039
- PMCID: PMC4000104
- DOI: 10.1128/EC.00243-13
Two-component histidine phosphotransfer protein Ypd1 is not essential for viability in Candida albicans
Abstract
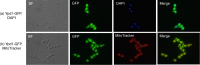
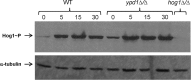
Prokaryotes and lower eukaryotes, such as yeasts, utilize two-component signal transduction pathways to adapt cells to environmental stress and to regulate the expression of genes associated with virulence. One of the central proteins in this type of signaling mechanism is the phosphohistidine intermediate protein Ypd1. Ypd1 is reported to be essential for viability in the model yeast Saccharomyces cerevisiae. We present data here showing that this is not the case for Candida albicans. Disruption of YPD1 causes cells to flocculate and filament constitutively under conditions that favor growth in yeast form. To determine the function of Ypd1 in the Hog1 mitogen-activated protein kinase (MAPK) pathway, we measured phosphorylation of Hog1 MAPK in ypd1Δ/Δ and wild-type strains of C. albicans. Constitutive phosphorylation of Hog1 was observed in the ypd1Δ/Δ strain compared to the wild-type strain. Furthermore, fluorescence microscopy revealed that green fluorescent protein (GFP)-tagged Ypd1 is localized to both the nucleus and the cytoplasm. The subcellular segregation of GFP-tagged Ypd1 hints at an important role(s) of Ypd1 in regulation of Ssk1 (cytosolic) and Skn7 (nuclear) response regulator proteins via phosphorylation in C. albicans. Overall, our findings have profound implications for a mechanistic understanding of two-component signaling pathways in C. albicans, and perhaps in other pathogenic fungi.
Figures





Similar articles
-
Blocking two-component signalling enhances Candida albicans virulence and reveals adaptive mechanisms that counteract sustained SAPK activation.PLoS Pathog. 2017 Jan 30;13(1):e1006131. doi: 10.1371/journal.ppat.1006131. eCollection 2017 Jan. PLoS Pathog. 2017. PMID: 28135328 Free PMC article.
-
Identification of YPD1, a gene of Candida albicans which encodes a two-component phosphohistidine intermediate protein.Yeast. 2000 Aug;16(11):1053-9. doi: 10.1002/1097-0061(200008)16:11<1053::AID-YEA598>3.0.CO;2-H. Yeast. 2000. PMID: 10923027
-
Effects of osmolytes on the SLN1-YPD1-SSK1 phosphorelay system from Saccharomyces cerevisiae.Biochemistry. 2009 Aug 25;48(33):8044-50. doi: 10.1021/bi900886g. Biochemistry. 2009. PMID: 19618914 Free PMC article.
-
Histidine phosphotransfer proteins in fungal two-component signal transduction pathways.Eukaryot Cell. 2013 Aug;12(8):1052-60. doi: 10.1128/EC.00083-13. Epub 2013 Jun 14. Eukaryot Cell. 2013. PMID: 23771905 Free PMC article. Review.
-
Stress-Activated Protein Kinases in Human Fungal Pathogens.Front Cell Infect Microbiol. 2019 Jul 17;9:261. doi: 10.3389/fcimb.2019.00261. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31380304 Free PMC article. Review.
Cited by
-
An array of signal-specific MoYpd1 isoforms determines full virulence in the pathogenic fungus Magnaporthe oryzae.Commun Biol. 2024 Mar 4;7(1):265. doi: 10.1038/s42003-024-05941-z. Commun Biol. 2024. PMID: 38438487 Free PMC article.
-
Role of the highly conserved G68 residue in the yeast phosphorelay protein Ypd1: implications for interactions between histidine phosphotransfer (HPt) and response regulator proteins.BMC Biochem. 2019 Jan 21;20(1):1. doi: 10.1186/s12858-019-0104-5. BMC Biochem. 2019. PMID: 30665347 Free PMC article.
-
Acetylation of BcHpt Lysine 161 Regulates Botrytis cinerea Sensitivity to Fungicides, Multistress Adaptation and Virulence.Front Microbiol. 2020 Jan 8;10:2965. doi: 10.3389/fmicb.2019.02965. eCollection 2019. Front Microbiol. 2020. PMID: 31969871 Free PMC article.
-
Transcriptional regulation of the caspofungin-induced cell wall damage response in Candida albicans.Curr Genet. 2020 Dec;66(6):1059-1068. doi: 10.1007/s00294-020-01105-8. Epub 2020 Sep 2. Curr Genet. 2020. PMID: 32876716 Free PMC article. Review.
-
The yeasts phosphorelay systems: a comparative view.World J Microbiol Biotechnol. 2017 Jun;33(6):111. doi: 10.1007/s11274-017-2272-z. Epub 2017 May 3. World J Microbiol Biotechnol. 2017. PMID: 28470426 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

