8-oxoguanine DNA glycosylase-1 augments proinflammatory gene expression by facilitating the recruitment of site-specific transcription factors
- PMID: 24489103
- PMCID: PMC3943862
- DOI: 10.4049/jimmunol.1302472
8-oxoguanine DNA glycosylase-1 augments proinflammatory gene expression by facilitating the recruitment of site-specific transcription factors
Abstract
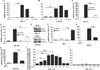
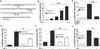
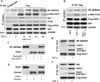
Among the insidious DNA base lesions, 8-oxo-7,8-dihydroguanine (8-oxoG) is one of the most abundant, a lesion that arises through the attack by reactive oxygen species on guanine, especially when located in cis-regulatory elements. 8-oxoG is repaired by the 8-oxoguanine glycosylase 1 (OGG1)-initiated DNA base excision repair pathway. In this study, we investigated whether 8-oxoG repair by OGG1 in promoter regions is compatible with a prompt gene expression and a host innate immune response. For this purpose, we used a mouse model of airway inflammation, supplemented with cell cultures, chromatin immunoprecipitation, small interfering RNA knockdown, real-time PCR, and comet and reporter transcription assays. Our data show that exposure of cells to TNF-α altered cellular redox, increased the 8-oxoG level in DNA, recruited OGG1 to promoter sequences, and transiently inhibited base excision repair of 8-oxoG. Promoter-associated OGG1 then enhanced NF-κB/RelA binding to cis-elements and facilitated recruitment of specificity protein 1, transcription initiation factor II-D, and p-RNA polymerase II, resulting in the rapid expression of chemokines/cytokines and inflammatory cell accumulation in mouse airways. Small interfering RNA depletion of OGG1 or prevention of guanine oxidation significantly decreased TNF-α-induced inflammatory responses. Taken together, these results show that nonproductive binding of OGG1 to 8-oxoG in promoter sequences could be an epigenetic mechanism to modulate gene expression for a prompt innate immune response.
Conflict of interest statement
There are no conflicts of interest
Figures




Similar articles
-
The role of 8-oxoguanine DNA glycosylase-1 in inflammation.Int J Mol Sci. 2014 Sep 23;15(9):16975-97. doi: 10.3390/ijms150916975. Int J Mol Sci. 2014. PMID: 25250913 Free PMC article. Review.
-
Oxidized Guanine Base Lesions Function in 8-Oxoguanine DNA Glycosylase-1-mediated Epigenetic Regulation of Nuclear Factor κB-driven Gene Expression.J Biol Chem. 2016 Dec 2;291(49):25553-25566. doi: 10.1074/jbc.M116.751453. Epub 2016 Oct 18. J Biol Chem. 2016. PMID: 27756845 Free PMC article.
-
Innate inflammation induced by the 8-oxoguanine DNA glycosylase-1-KRAS-NF-κB pathway.J Immunol. 2014 Nov 1;193(9):4643-53. doi: 10.4049/jimmunol.1401625. Epub 2014 Sep 29. J Immunol. 2014. PMID: 25267977 Free PMC article.
-
Oxidized base 8-oxoguanine, a product of DNA repair processes, contributes to dendritic cell activation.Free Radic Biol Med. 2019 Nov 1;143:209-220. doi: 10.1016/j.freeradbiomed.2019.08.010. Epub 2019 Aug 10. Free Radic Biol Med. 2019. PMID: 31408726 Free PMC article.
-
Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases.Free Radic Biol Med. 2017 Jun;107:179-201. doi: 10.1016/j.freeradbiomed.2016.11.042. Epub 2016 Nov 27. Free Radic Biol Med. 2017. PMID: 27903453 Review.
Cited by
-
OGG1 inhibition suppresses African swine fever virus replication.Virol Sin. 2023 Feb;38(1):96-107. doi: 10.1016/j.virs.2022.11.006. Epub 2022 Nov 23. Virol Sin. 2023. PMID: 36435451 Free PMC article.
-
Gene-Specific Assessment of Guanine Oxidation as an Epigenetic Modulator for Cardiac Specification of Mouse Embryonic Stem Cells.PLoS One. 2016 Jun 1;11(6):e0155792. doi: 10.1371/journal.pone.0155792. eCollection 2016. PLoS One. 2016. PMID: 27249188 Free PMC article.
-
Genetic Variations in Nucleotide Excision Repair Pathway Genes and Risk of Allergic Rhinitis.Mediators Inflamm. 2022 Jun 3;2022:7815283. doi: 10.1155/2022/7815283. eCollection 2022. Mediators Inflamm. 2022. PMID: 35693108 Free PMC article.
-
Inhibitors of DNA Glycosylases as Prospective Drugs.Int J Mol Sci. 2020 Apr 28;21(9):3118. doi: 10.3390/ijms21093118. Int J Mol Sci. 2020. PMID: 32354123 Free PMC article. Review.
-
The role of 8-oxoguanine DNA glycosylase-1 in inflammation.Int J Mol Sci. 2014 Sep 23;15(9):16975-97. doi: 10.3390/ijms150916975. Int J Mol Sci. 2014. PMID: 25250913 Free PMC article. Review.
References
-
- Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. - PubMed
-
- Steenken S, Jovanovic SV. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 1997;119:617–618.
-
- Dizdaroglu M. Formation of an 8-hydroxyguanine moiety in deoxyribonucleic acid on gamma-irradiation in aqueous solution. Biochemistry. 1985;24:4476–4481. - PubMed
-
- Nishimura S. Involvement of mammalian OGG1(MMH) in excision of the 8-hydroxyguanine residue in DNA. Free Radic Biol Med. 2002;32:813–821. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

