Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis
- PMID: 24489112
- PMCID: PMC3934721
- DOI: 10.1194/jlr.R045559
Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis
Abstract
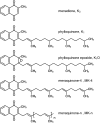
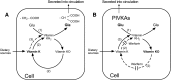
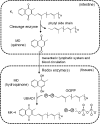
In contrast to other fat-soluble vitamins, dietary vitamin K is rapidly lost to the body resulting in comparatively low tissue stores. Deficiency is kept at bay by the ubiquity of vitamin K in the diet, synthesis by gut microflora in some species, and relatively low vitamin K cofactor requirements for γ-glutamyl carboxylation. However, as shown by fatal neonatal bleeding in mice that lack vitamin K epoxide reductase (VKOR), the low requirements are dependent on the ability of animals to regenerate vitamin K from its epoxide metabolite via the vitamin K cycle. The identification of the genes encoding VKOR and its paralog VKOR-like 1 (VKORL1) has accelerated understanding of the enzymology of this salvage pathway. In parallel, a novel human enzyme that participates in the cellular conversion of phylloquinone to menaquinone (MK)-4 was identified as UbiA prenyltransferase-containing domain 1 (UBIAD1). Recent studies suggest that side-chain cleavage of oral phylloquinone occurs in the intestine, and that menadione is a circulating precursor of tissue MK-4. The mechanisms and functions of vitamin K recycling and MK-4 synthesis have dominated advances made in vitamin K biochemistry over the last five years and, after a brief overview of general metabolism, are the main focuses of this review.
Keywords: UbiA prenyltransferase-containing domain 1; anticoagulants; menaquinones; phylloquinone; vitamin K antagonists; vitamin K dehydrogenase; vitamin K epoxide; vitamin K epoxide reductase; warfarin; warfarin resistance; γ-carboxyglutamate proteins.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

