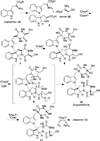
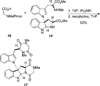
Studies on the Biosynthesis of Chetomin: Enantiospecific Synthesis of a Putative, Late-Stage Biosynthetic Intermediate
- PMID: 24489414
- PMCID: PMC3908826
- DOI: 10.1016/j.tet.2012.10.075
Studies on the Biosynthesis of Chetomin: Enantiospecific Synthesis of a Putative, Late-Stage Biosynthetic Intermediate
Abstract
The enantiospecific synthesis of desthiochetomin, a putative biosynthetic intermediate of the epidithiodioxopiperazine natural product chetomin, is described. A diastereoselective N-alkylation was employed to form the key C3-N1' bond of the heterodimeric indoline core, followed by peptide coupling and dioxopiperazine cyclization with the requisite N-methyl amino acids. A related sarcosine-derived dioxopiperazine was prepared in the same manner. The first proposed biosynthesis of chetomin is also detailed in the text.
Keywords: Aspergillus versicolor; biosynthesis; prenylated indole alkaloid.
Figures
Similar articles
-
On Demand Synthesis of C3-N1' Bisindoles by a Formal Umpolung Strategy: First Total Synthesis of (±)-Rivularin A.Chemistry. 2024 Feb 21;30(11):e202302963. doi: 10.1002/chem.202302963. Epub 2024 Jan 8. Chemistry. 2024. PMID: 37988219
-
Molecular diversity via amino acid derived alpha-amino nitriles: synthesis of spirocyclic 2,6-dioxopiperazine derivatives.J Org Chem. 2005 Apr 29;70(9):3660-6. doi: 10.1021/jo050146m. J Org Chem. 2005. PMID: 15845004
-
Synergism between genome sequencing, tandem mass spectrometry and bio-inspired synthesis reveals insights into nocardioazine B biogenesis.Org Biomol Chem. 2015 Jul 14;13(26):7177-92. doi: 10.1039/c5ob00537j. Epub 2015 May 29. Org Biomol Chem. 2015. PMID: 26022437
-
Structural and stereochemical diversity in prenylated indole alkaloids containing the bicyclo[2.2.2]diazaoctane ring system from marine and terrestrial fungi.Nat Prod Rep. 2018 Jun 20;35(6):532-558. doi: 10.1039/c7np00042a. Nat Prod Rep. 2018. PMID: 29632911 Free PMC article. Review.
-
Prenylated indole derivatives from fungi: structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis.Nat Prod Rep. 2010 Jan;27(1):57-78. doi: 10.1039/b909987p. Epub 2009 Nov 19. Nat Prod Rep. 2010. PMID: 20024094 Review.
Cited by
-
Double the Chemistry, Double the Fun: Structural Diversity and Biological Activity of Marine-Derived Diketopiperazine Dimers.Mar Drugs. 2019 Sep 27;17(10):551. doi: 10.3390/md17100551. Mar Drugs. 2019. PMID: 31569621 Free PMC article. Review.
-
Cytotoxic and antimicrobial indole alkaloids from an endophytic fungus Chaetomium sp. SYP-F7950 of Panax notoginseng.RSC Adv. 2019 Sep 12;9(49):28754-28763. doi: 10.1039/c9ra04747f. eCollection 2019 Sep 9. RSC Adv. 2019. PMID: 35529647 Free PMC article.
-
Epipolythiodioxopiperazine-Based Natural Products: Building Blocks, Biosynthesis and Biological Activities.Chembiochem. 2022 Dec 5;23(23):e202200341. doi: 10.1002/cbic.202200341. Epub 2022 Sep 15. Chembiochem. 2022. PMID: 35997236 Free PMC article. Review.
-
Evaluation of the Chetomin effect on histopathological features in a murine acute spinal cord injury model.World Neurosurg X. 2024 Sep 26;25:100414. doi: 10.1016/j.wnsx.2024.100414. eCollection 2025 Jan. World Neurosurg X. 2024. PMID: 39411272 Free PMC article.
-
Concise total synthesis of (+)-asperazine A and (+)-pestalazine B.Org Biomol Chem. 2018 Jan 3;16(2):202-207. doi: 10.1039/c7ob02985c. Org Biomol Chem. 2018. PMID: 29243756 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous