Efficacy of indacaterol on quality of life and pulmonary function in patients with COPD and inhaler device preferences
- PMID: 24489464
- PMCID: PMC3904808
- DOI: 10.2147/COPD.S56777
Efficacy of indacaterol on quality of life and pulmonary function in patients with COPD and inhaler device preferences
Abstract
Background: Indacaterol is a novel, once-daily, inhaled, long-acting b2-agonist for patients with chronic obstructive pulmonary disease (COPD). The study objective was to evaluate the efficacy of indacaterol on quality of life and pulmonary function in patients with COPD in a real-world setting, and also to evaluate its inhaler device (Breezhaler®), which is important for both adherence and management.
Methods: Twenty-eight outpatients with COPD were treated with indacaterol (150 μg once daily for 8 weeks), and the effects on pulmonary function were evaluated using a questionnaire survey with the modified Medical Research Council (mMRC) dyspnea scale and COPD assessment test (CAT) before and after treatment. Similar investigations were also performed separately among different baseline medications. Moreover, original questionnaire surveys for indacaterol and its device were performed.
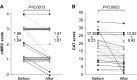
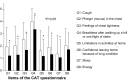
Results: Overall, mMRC dyspnea scale and CAT scores significantly improved (1.96±1.04 to 1.57±1.07 and 17.39±8.23 to 12.82±8.42, respectively; P<0.05). Significant improvements in forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were also observed on pulmonary function tests (2.91±0.66 L to 3.07±0.65 L and 1.46±0.60 L to 1.58±0.59 L, respectively; P<0.05). Replacement therapy from salmeterol to indacaterol significantly improved mMRC and FVC values, but did not significantly improve CAT scores or other pulmonary functions. Add-on therapy with indacaterol significantly improved mMRC score, CAT score, FVC, and FEV1, regardless of whether tiotropium was used as a baseline treatment. All subjects in a questionnaire survey found the inhaler device easy to use. There were no serious adverse events leading to treatment discontinuation.
Conclusion: Indacaterol is thought to be effective and well tolerated as a bronchodilator for the management of COPD. Treatment with indacaterol in addition to a long-acting muscarinic antagonist was also useful.
Keywords: COPD; device; indacaterol; quality of life; respiratory function.
Figures

Similar articles
-
Efficacy and safety of coadministration of once-daily indacaterol and glycopyrronium versus indacaterol alone in COPD patients: the GLOW6 study.Int J Chron Obstruct Pulmon Dis. 2014 Feb 24;9:215-28. doi: 10.2147/COPD.S51592. eCollection 2014. Int J Chron Obstruct Pulmon Dis. 2014. PMID: 24596459 Free PMC article. Clinical Trial.
-
Real-life effectiveness of indacaterol-glycopyrronium after switching from tiotropium or salmeterol/fluticasone therapy in patients with symptomatic COPD: the POWER study.Int J Chron Obstruct Pulmon Dis. 2019 Jan 18;14:249-260. doi: 10.2147/COPD.S185485. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 30718952 Free PMC article. Clinical Trial.
-
The impact of indacaterol/glycopyrronium fixed-dose combination versus tiotropium monotherapy on lung function and treatment preference: a randomized crossover study - the FAVOR study.Int J Chron Obstruct Pulmon Dis. 2017 Dec 22;13:69-77. doi: 10.2147/COPD.S146189. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 29317812 Free PMC article. Clinical Trial.
-
Role of combined indacaterol and glycopyrronium bromide (QVA149) for the treatment of COPD in Japan.Int J Chron Obstruct Pulmon Dis. 2015 Apr 21;10:813-22. doi: 10.2147/COPD.S56067. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 25960646 Free PMC article. Review.
-
Safety and efficacy of 12-week or longer indacaterol treatment in moderate-to-severe COPD patients: a systematic review.Lung. 2013 Apr;191(2):135-46. doi: 10.1007/s00408-012-9444-2. Epub 2013 Jan 10. Lung. 2013. PMID: 23306410
Cited by
-
Patient preferences in severe COPD and asthma: a comprehensive literature review.Int J Chron Obstruct Pulmon Dis. 2015 Apr 8;10:739-44. doi: 10.2147/COPD.S82179. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 25914530 Free PMC article. Review.
-
A real-world evaluation of indacaterol and other bronchodilators in COPD: the INFLOW study.Int J Chron Obstruct Pulmon Dis. 2015 Oct 5;10:2109-20. doi: 10.2147/COPD.S83071. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26491281 Free PMC article.
-
A comprehensive assessment using COPD assessment test scoring and modified Medical Research Council dyspnea scoring is necessary for personalized therapy for COPD patients.Int J Chron Obstruct Pulmon Dis. 2015 Oct 15;10:2203-6. doi: 10.2147/COPD.S94509. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26508850 Free PMC article. No abstract available.
-
Patient considerations in the treatment of COPD: focus on the new combination inhaler umeclidinium/vilanterol.Patient Prefer Adherence. 2015 Feb 2;9:235-42. doi: 10.2147/PPA.S71535. eCollection 2015. Patient Prefer Adherence. 2015. PMID: 25673975 Free PMC article. Review.
-
Predicting treatable traits for long-acting bronchodilators in patients with stable COPD.Int J Chron Obstruct Pulmon Dis. 2017 Dec 12;12:3557-3565. doi: 10.2147/COPD.S151909. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 29263660 Free PMC article. Clinical Trial.
References
-
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. - PubMed
-
- Gershon AS, Warner L, Cascagnette P, Victor JC, To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011;378:991–996. - PubMed
-
- Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107:1481–1490. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

