Missing links in antibody assembly control
- PMID: 24489546
- PMCID: PMC3893805
- DOI: 10.1155/2013/606703
Missing links in antibody assembly control
Abstract
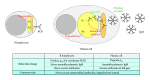
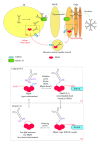
Fidelity of the humoral immune response requires that quiescent B lymphocytes display membrane bound immunoglobulin M (IgM) on B lymphocytes surface as part of the B cell receptor, whose function is to recognize an antigen. At the same time B lymphocytes should not secrete IgM until recognition of the antigen has occurred. The heavy chains of the secretory IgM have a C-terminal tail with a cysteine instead of a membrane anchor, which serves to covalently link the IgM subunits by disulfide bonds to form "pentamers" or "hexamers." By virtue of the same cysteine, unassembled secretory IgM subunits are recognized and retained (via mixed disulfide bonds) by members of the protein disulfide isomerase family, in particular ERp44. This so-called "thiol-mediated retention" bars assembly intermediates from prematurely leaving the cell and thereby exerts quality control on the humoral immune response. In this essay we discuss recent findings on how ERp44 governs such assembly control in a pH-dependent manner, shuttling between the cisGolgi and endoplasmic reticulum, and finally on how pERp1/MZB1, possibly as a co-chaperone of GRP94, may help to overrule the thiol-mediated retention in the activated B cell to give way to antibody secretion.
Figures


References
-
- Hendershot LM, Sitia R. Immunoglobulin assembly and secretion. In: Honjo T, Alt FW, Neuberger MS, editors. Molecular Biology of B Cells. Amsterdam, The Netherlands: Elsevier Academic Press; 2005. pp. 261–273.
-
- Sitia R, Neuberger M, Alberini C, et al. Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell. 1990;60(5):781–790. - PubMed
-
- Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annual Review of Biochemistry. 2011;80:71–99. - PubMed
-
- Haas IG, Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983;306(5941):387–389. - PubMed
-
- Melnick J, Aviel S, Argon Y. The endoplasmic reticulum stress protein GRP94, in addition to BiP, associates with unassembled immunoglobulin chains. Journal of Biological Chemistry. 1992;267(30):21303–21306. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

