Pivoting between calmodulin lobes triggered by calcium in the Kv7.2/calmodulin complex
- PMID: 24489773
- PMCID: PMC3904923
- DOI: 10.1371/journal.pone.0086711
Pivoting between calmodulin lobes triggered by calcium in the Kv7.2/calmodulin complex
Abstract
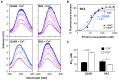
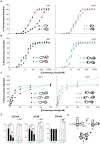
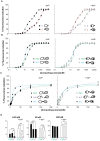
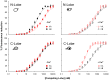
Kv7.2 (KCNQ2) is the principal molecular component of the slow voltage gated M-channel, which strongly influences neuronal excitability. Calmodulin (CaM) binds to two intracellular C-terminal segments of Kv7.2 channels, helices A and B, and it is required for exit from the endoplasmic reticulum. However, the molecular mechanisms by which CaM controls channel trafficking are currently unknown. Here we used two complementary approaches to explore the molecular events underlying the association between CaM and Kv7.2 and their regulation by Ca(2+). First, we performed a fluorometric assay using dansylated calmodulin (D-CaM) to characterize the interaction of its individual lobes to the Kv7.2 CaM binding site (Q2AB). Second, we explored the association of Q2AB with CaM by NMR spectroscopy, using (15)N-labeled CaM as a reporter. The combined data highlight the interdependency of the N- and C-lobes of CaM in the interaction with Q2AB, suggesting that when CaM binds Ca(2+) the binding interface pivots between the N-lobe whose interactions are dominated by helix B and the C-lobe where the predominant interaction is with helix A. In addition, Ca(2+) makes CaM binding to Q2AB more difficult and, reciprocally, the channel weakens the association of CaM with Ca(2+).
Conflict of interest statement
Figures
References
-
- Jurado LA, Chockalingam PS, Jarrett HW (1999) Apocalmodulin. Physiol Rev 79 661–682. - PubMed
-
- Hoeflich KP, Ikura M (2002) Calmodulin in action: diversity in target recognition and activation mechanisms. Cell 108 739–742. - PubMed
-
- Linse S, Helmersson A, Forsen S (1991) Calcium binding to calmodulin and its globular domains. J Biol Chem 266 8050–8054. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

