3 β-hydroxysteroid-Δ 24 reductase (DHCR24) protects neuronal cells from apoptotic cell death induced by endoplasmic reticulum (ER) stress
- PMID: 24489783
- PMCID: PMC3906068
- DOI: 10.1371/journal.pone.0086753
3 β-hydroxysteroid-Δ 24 reductase (DHCR24) protects neuronal cells from apoptotic cell death induced by endoplasmic reticulum (ER) stress
Abstract
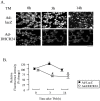
3β-Hydroxysteroid-Δ24 reductase (DHCR24) is an endoplasmic reticulum (ER)-localized multifunctional enzyme that possesses anti-apoptotic and cholesterol-synthesizing activities. Accumulating evidence suggests that ER stress is involved in the pathogenesis of neurodegenerative disease. In this study, we investigated whether DHCR24 may function as a neuroprotective protein under ER stress. Neuroblastoma N2A cells were infected with adenovirus expressing myc-tagged DHCR24 (Ad-DHCR24) or lacZ (Ad-lacZ, serving as a control) and subjected to ER-stress, induced with Tunicamycin (TM). Cells infected with Ad-DHCR24-myc were resistant to TM-induced apoptosis, and showed weaker level of caspase-12 activity. These cells also exhibited lower levels of Bip and CHOP proteins than Ad-LacZ-infected cells. Moreover, a stronger and rapid activation of PERK, and a prolonged activation of JNK and p38 were observed in Ad-LacZ-infected cells. The generation of intracellular reactive oxygen species from ER stress was also diminished by the overexpression of DHCR24. Additionally, intracellular cholesterol level was also elevated in the Ad-DHCR24-infected cells, accompanied by a well-organized formation of caveolae (cholesterol-rich microdomain) on the plasma membrane, and improved colocalization of caveolin-1 and insulin-like growth factor 1 receptor. These results demonstrated for the first time that DHCR24 could protect neuronal cells from apoptosis induced by ER stress.
Conflict of interest statement
Figures






References
-
- Lai E, Teodoro T, Volchuk A (2007) Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology.(Bethesda.) 22, 193–201. - PubMed
-
- Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat.Rev.Mol.Cell Biol. 13, 89–102. - PubMed
-
- Yoshida H (2007) ER stress and diseases. FEBS J. 274, 630–658. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

