Mathematical modeling of renal tubular glucose absorption after glucose load
- PMID: 24489817
- PMCID: PMC3906102
- DOI: 10.1371/journal.pone.0086963
Mathematical modeling of renal tubular glucose absorption after glucose load
Abstract
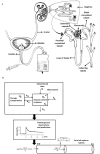
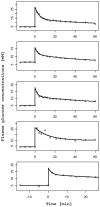
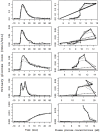
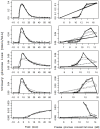
A partial differential Progressive Tubular Reabsorption (PTR) model, describing renal tubular glucose reabsorption and urinary glucose excretion following a glucose load perturbation, is proposed and fitted to experimental data from five subjects. For each subject the Glomerular Filtration Rate was estimated and both blood and urine glucose were sampled following an Intra-Venous glucose bolus. The PTR model was compared with a model representing the conventional Renal Threshold Hypothesis (RTH). A delay bladder compartment was introduced in both formulations. For the RTH model, the average threshold for glycosuria varied between 9.90 ± 4.50 mmol/L and 10.63 ± 3.64 mmol/L (mean ± Standard Deviation) under different hypotheses; the corresponding average maximal transport rates varied between 0.48 ± 0.45 mmol/min (86.29 ± 81.22 mg/min) and 0.50 ± 0.42 mmol/min (90.62 ± 76.15 mg/min). For the PTR Model, the average maximal transports rates varied between 0.61 ± 0.52 mmol/min (109.57 ± 93.77 mg/min) and 0.83 ± 0.95 mmol/min (150.13 ± 171.85 mg/min). The time spent by glucose inside the tubules before entering the bladder compartment varied between 1.66 ± 0.73 min and 2.45 ± 1.01 min. The PTR model proved much better than RTH at fitting observations, by correctly reproducing the delay of variations of glycosuria with respect to the driving glycemia, and by predicting non-zero urinary glucose elimination at low glycemias. This model is useful when studying both transients and steady-state glucose elimination as well as in assessing drug-related changes in renal glucose excretion.
Conflict of interest statement
Figures
References
-
- Triplitt CL (2012) Examining the mechanisms of glucose regulation. Am J Manag Care 18: S4–10 44183 [pii]. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

