Sodium ferulate inhibits neointimal hyperplasia in rat balloon injury model
- PMID: 24489938
- PMCID: PMC3906191
- DOI: 10.1371/journal.pone.0087561
Sodium ferulate inhibits neointimal hyperplasia in rat balloon injury model
Abstract
Background/aim: Neointimal formation after vessel injury is a complex process involving multiple cellular and molecular processes. Inhibition of intimal hyperplasia plays an important role in preventing proliferative vascular diseases, such as restenosis. In this study, we intended to identify whether sodium ferulate could inhibit neointimal formation and further explore potential mechanisms involved.
Methods: Cultured vascular smooth muscle cells (VSMCs) isolated from rat thoracic aorta were pre-treated with 200 µmol/L sodium ferulate for 1 hour and then stimulated with 1 µmol/L angiotensin II (Ang II) for 1 hour or 10% serum for 48 hours. Male Sprague-Dawley rats subjected to balloon catheter insertion were administrated with 200 mg/kg sodium ferulate (or saline) for 7 days before sacrificed.
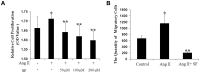
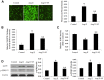
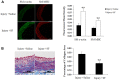
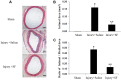
Results: In presence of sodium ferulate, VSMCs exhibited decreased proliferation and migration, suppressed intracellular reactive oxidative species production and NADPH oxidase activity, increased SOD activation and down-regulated p38 phosphorylation compared to Ang II-stimulated alone. Meanwhile, VSMCs treated with sodium ferulate showed significantly increased protein expression of smooth muscle α-actin and smooth muscle myosin heavy chain protein. The components of Notch pathway, including nuclear Notch-1 protein, Jagged-1, Hey-1 and Hey-2 mRNA, as well as total β-catenin protein and Cyclin D1 mRNA of Wnt signaling, were all significantly decreased by sodium ferulate in cells under serum stimulation. The levels of serum 8-iso-PGF2α and arterial collagen formation in vessel wall were decreased, while the expression of contractile markers was increased in sodium ferulate treated rats. A decline of neointimal area, as well as lower ratio of intimal to medial area was observed in sodium ferulate group.
Conclusion: Sodium ferulate attenuated neointimal hyperplasia through suppressing oxidative stress and phenotypic switching of VSMCs.
Conflict of interest statement
Figures
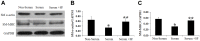


Similar articles
-
Naringenin inhibits angiotensin II-induced vascular smooth muscle cells proliferation and migration and decreases neointimal hyperplasia in balloon injured rat carotid arteries through suppressing oxidative stress.Biol Pharm Bull. 2013;36(10):1549-55. doi: 10.1248/bpb.b13-00247. Epub 2013 Aug 3. Biol Pharm Bull. 2013. PMID: 23912743
-
Inhibition of neointimal hyperplasia in the rat carotid artery injury model by a HMGB1 inhibitor.Atherosclerosis. 2012 Oct;224(2):332-9. doi: 10.1016/j.atherosclerosis.2012.07.020. Epub 2012 Jul 24. Atherosclerosis. 2012. PMID: 22857898
-
Resveratrol inhibits phenotypic switching of neointimal vascular smooth muscle cells after balloon injury through blockade of Notch pathway.J Cardiovasc Pharmacol. 2014 Mar;63(3):233-9. doi: 10.1097/FJC.0000000000000040. J Cardiovasc Pharmacol. 2014. PMID: 24603118
-
Apoptosis and Cell Proliferation Markers in Inflammatory-Fibroproliferative Diseases of the Vessel Wall (Review).Sovrem Tekhnologii Med. 2021;12(4):119-126. doi: 10.17691/stm2020.12.4.13. Epub 2020 Aug 27. Sovrem Tekhnologii Med. 2021. PMID: 34795999 Free PMC article. Review.
-
The Exacerbating Effects of the Tumor Necrosis Factor in Cardiovascular Stenosis: Intimal Hyperplasia.Cancers (Basel). 2024 Apr 8;16(7):1435. doi: 10.3390/cancers16071435. Cancers (Basel). 2024. PMID: 38611112 Free PMC article. Review.
Cited by
-
Clinical effects of perazine ferulate tablets combined with eucalyptol limonene pinene enteric soft capsules for treatment of children with IgA nephropathy.Exp Ther Med. 2016 Jul;12(1):169-172. doi: 10.3892/etm.2016.3312. Epub 2016 May 9. Exp Ther Med. 2016. PMID: 27347034 Free PMC article.
References
-
- Kibos A, Campeanu A, Tintoiu I (2007) Pathophysiology of coronary artery in-stent restenosis. Acute Card Care 9: 111–119. - PubMed
-
- Khan W, Farah S, Domb AJ (2012) Drug eluting stents: developments and current status. J Control Release 161: 703–712. - PubMed
-
- Alahmar AE, Grayson AD, Andron M, Egred M, Roberts ED, et al. (2009) Reduction in mortality and target-lesion revascularisation at 2 years: a comparison between drug-eluting stents and conventional bare-metal stents in the "real world". Int J Cardiol 132: 398–404. - PubMed
-
- Papafaklis MI, Chatzizisis YS, Naka KK, Giannoglou GD, Michalis LK (2012) Drug-eluting stent restenosis: effect of drug type, release kinetics, hemodynamics and coating strategy. Pharmacol Ther 134: 43–53. - PubMed
-
- De Labriolle A, Bonello L, Lemesle G, Steinberg DH, Roy P, et al. (2009) Clinical presentation and outcome of patients hospitalized for symptomatic in-stent restenosis treated by percutaneous coronary intervention: comparison between drug-eluting stents and bare-metal stents. Arch Cardiovasc Dis 102: 209–217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

