Transplantation of Fas-deficient or wild-type neural stem/progenitor cells (NPCs) is equally efficient in treating experimental autoimmune encephalomyelitis (EAE)
- PMID: 24489991
- PMCID: PMC3902222
Transplantation of Fas-deficient or wild-type neural stem/progenitor cells (NPCs) is equally efficient in treating experimental autoimmune encephalomyelitis (EAE)
Abstract
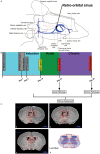
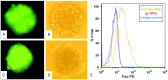
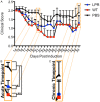
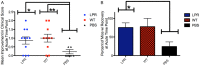
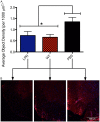
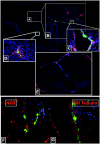
Studies have shown that neural stem/progenitor cell (NPC) transplantation is beneficial in experimental autoimmune encephalomyelitis (EAE), an established animal model of multiple sclerosis (MS). It is unclear whether NPCs have the ability to integrate into the host CNS to replace lost cells or if their main mechanism of action is via bystander immunomodulation. Understanding the mechanisms by which NPCs exert their beneficial effects as well as exploring methods to increase post-transplantation survival and differentiation is critical to advancing this treatment strategy. Using the EAE model and Fas-deficient (lpr) NPCs, we investigated the effects of altering the Fas system in NPC transplantation therapy. We show that transplantation of NPCs into EAE mice ameliorates clinical symptoms with greater efficacy than sham treatments regardless of cell type (wt or lpr). NPC transplantation via retro-orbital injections significantly decreased inflammatory infiltrates at the acute time point, with a similar trend at the chronic time point. Both wt and lpr NPCs injected into mice with EAE were able to home to sites of CNS inflammation in the periventricular brain and lumbar spinal cord. Both wt and lpr NPCs have the same capacity for inducing apoptosis of Th1 and Th17 cells, and minimal numbers of NPCs entered the CNS. These cells did not express terminal differentiation markers, suggesting that NPCs exert their effects mainly via bystander peripheral immunomodulation.
Keywords: Experimental autoimmune encephalomyelitis (EAE); Fas-deficient (lpr) NPC; neural progenitor cells (NPC); neuroprotection.
Figures
References
-
- Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. - PubMed
-
- Galli R, Gritti A, Bonfanti L, Vescovi AL. Neural stem cells: an overview. Circ Res. 2003;92:598–608. - PubMed
-
- Einstein O, Karussis D, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Abramsky O, Ben-Hur T. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol Cell Neurosci. 2003;24:1074–1082. - PubMed
-
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. - PubMed
-
- Politi LS, Bacigaluppi M, Brambilla E, Cadioli M, Falini A, Comi G, Scotti G, Martino G, Pluchino S. Magnetic-resonance-based tracking and quantification of intravenously injected neural stem cell accumulation in the brains of mice with experimental multiple sclerosis. Stem Cells. 2007;25:2583–2592. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
