Cytochrome P450-Catalyzed Insertion of Carbenoids into N-H Bonds
- PMID: 24490022
- PMCID: PMC3906682
- DOI: 10.1039/C3SC52535J
Cytochrome P450-Catalyzed Insertion of Carbenoids into N-H Bonds
Abstract
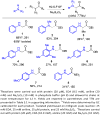
Expanding nature's catalytic repertoire to include reactions important in synthetic chemistry will open new opportunities for 'green' chemistry and biosynthesis. We demonstrate enzyme-catalyzed insertion of carbenoids into N-H bonds. This type of bond disconnection, which has no counterpart in nature, can be mediated by variants of the cytochrome P450 from Bacillus megaterium. The N-H insertion reaction takes place in water, provides the desired products in 26-83% yield, forms the single addition product exclusively, and does not require slow addition of the diazo component.
Figures
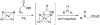


Similar articles
-
Intramolecular cyclopropanation and C-H insertion reactions with metal carbenoids generated from cyclopropenes.Acc Chem Res. 2015 Apr 21;48(4):1021-31. doi: 10.1021/acs.accounts.5b00016. Epub 2015 Mar 12. Acc Chem Res. 2015. PMID: 25763601
-
Nature's Machinery, Repurposed: Expanding the Repertoire of Iron-Dependent Oxygenases.ACS Catal. 2020 Oct 16;10(20):12239-12255. doi: 10.1021/acscatal.0c03606. Epub 2020 Sep 28. ACS Catal. 2020. PMID: 33282461 Free PMC article.
-
Olefin cyclopropanation via carbene transfer catalyzed by engineered cytochrome P450 enzymes.Science. 2013 Jan 18;339(6117):307-10. doi: 10.1126/science.1231434. Epub 2012 Dec 20. Science. 2013. PMID: 23258409
-
Navigating the Unnatural Reaction Space: Directed Evolution of Heme Proteins for Selective Carbene and Nitrene Transfer.Acc Chem Res. 2021 Mar 2;54(5):1209-1225. doi: 10.1021/acs.accounts.0c00591. Epub 2021 Jan 25. Acc Chem Res. 2021. PMID: 33491448 Free PMC article. Review.
-
Recent advances in transition-metal-catalyzed carbene insertion to C-H bonds.Chem Soc Rev. 2022 Apr 4;51(7):2759-2852. doi: 10.1039/d1cs00895a. Chem Soc Rev. 2022. PMID: 35297455 Review.
Cited by
-
Directed Evolution of P450 BM3 towards Functionalization of Aromatic O-Heterocycles.Int J Mol Sci. 2019 Jul 8;20(13):3353. doi: 10.3390/ijms20133353. Int J Mol Sci. 2019. PMID: 31288417 Free PMC article.
-
Catalytic iron-carbene intermediate revealed in a cytochrome c carbene transferase.Proc Natl Acad Sci U S A. 2018 Jul 10;115(28):7308-7313. doi: 10.1073/pnas.1807027115. Epub 2018 Jun 26. Proc Natl Acad Sci U S A. 2018. PMID: 29946033 Free PMC article.
-
Chemoselective Cyclopropanation over Carbene Y-H Insertion Catalyzed by an Engineered Carbene Transferase.J Org Chem. 2018 Jul 20;83(14):7480-7490. doi: 10.1021/acs.joc.8b00946. Epub 2018 Jul 6. J Org Chem. 2018. PMID: 29905476 Free PMC article.
-
An Enzymatic Platform for the Highly Enantioselective and Stereodivergent Construction of Cyclopropyl-δ-lactones.Angew Chem Int Ed Engl. 2020 Nov 23;59(48):21634-21639. doi: 10.1002/anie.202007953. Epub 2020 Sep 17. Angew Chem Int Ed Engl. 2020. PMID: 32667122 Free PMC article.
-
Carbene Transfer Reactions Catalysed by Dyes of the Metalloporphyrin Group.Molecules. 2018 Mar 29;23(4):792. doi: 10.3390/molecules23040792. Molecules. 2018. PMID: 29596367 Free PMC article. Review.
References
-
- Yates PJ. J Am Chem Soc. 1952;74:5376.
- Saegusa T, Ito Y, Kobayashi S, Hirota K, Shimizu T. Tetrahedron Lett. 1966;49:6131.
- Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds. Wiley; New York: 1998. Chapter 8.2.
-
- Bolm C, Kasyan A, Drauz K, Gunther K, Raabe G. Angew Chem Int Ed. 2000;39:2288. - PubMed
- Davis F, Fang T, Goswami R. Org Lett. 2002;4:1599. - PubMed
- Yamazaki K, Kondo Y. Chem Commun. 2002;38:210. - PubMed
- Bashford KE, Cooper AL, Kane PD, Moody CJ, Muthusamy S, Swann E. J Chem Soc, Perkin Trans. 2002;1:1672.
- Lee SH, Clapham B, Koch G, Zimmerman J, Janda KD. J Comb Chem. 2003;5:188. - PubMed
- Burtoloso ACB, Correia CRD. Tetrahedron Lett. 2004;45:3355.
- Davies JR, Kane PD, Moody CJ. Tetrahedron. 2004;60:3967.
- Matsushita H, Lee SH, Yoshida K, Clapham B, Koch G, Zimmerman J, Janda K. Org Lett. 2004;6:4627. - PubMed
- Aviv I, Gross Z. Chem Commun. 2006:4477. - PubMed
- Liu B, Zhu SF, Zhang W, Chen C, Zhou QL. J Am Chem Soc. 2007;129:5834. - PubMed
-
- Ratcliffe R, Salzmann T, Christensen B. Tetrahedron Lett. 1980;21:31.
- Salzmann TN, Ratcliffe RW, Christensen BG, Bouffard FA. J Am Chem Soc. 1980;102:6163. - PubMed
-
- Zhu SF, Xu B, Wang GP, Zhou QL. J Am Chem Soc. 2012;134:436. - PubMed
- Anding BJ, Woo LK. Organometallics. 2013;32:2599–2607.
-
-
For selected recent examples see: Bachmann S, Fielenbach D, Jorgensen KA. Org Biomol Chem. 2004;2:3044.Lee EC, Fu GC. J Am Chem Soc. 2007;129:12066.Morilla ME, Díaz-Requejo MM, Belderrain TR, Nicasio MC, Trofimenko S, Pérez P. Chem Commun. 2002:2998.Moyer M, Feldman P, Rapoport H. J Org Chem. 1985;50:5223.Kantam M, Neelima B, Reddy C. J Mol Cat A: Chemical. 2006;256:269.Shang, Wang J. Tetrahedron. 2008;64:6577.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

