Several affinity tags commonly used in chromatographic purification
- PMID: 24490106
- PMCID: PMC3893739
- DOI: 10.1155/2013/581093
Several affinity tags commonly used in chromatographic purification
Abstract
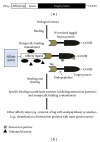
Affinity tags have become powerful tools from basic biological research to structural and functional proteomics. They were widely used to facilitate the purification and detection of proteins of interest, as well as the separation of protein complexes. Here, we mainly discuss the benefits and drawbacks of several affinity or epitope tags frequently used, including hexahistidine tag, FLAG tag, Strep II tag, streptavidin-binding peptide (SBP) tag, calmodulin-binding peptide (CBP), glutathione S-transferase (GST), maltose-binding protein (MBP), S-tag, HA tag, and c-Myc tag. In some cases, a large-size affinity tag, such as GST or MBP, can significantly impact on the structure and biological activity of the fusion partner protein. So it is usually necessary to excise the tag by protease. The most commonly used endopeptidases are enterokinase, factor Xa, thrombin, tobacco etch virus, and human rhinovirus 3C protease. The proteolysis features of these proteases are described in order to provide a general guidance on the proteolytic removal of the affinity tags.
Figures
References
-
- Arnau J, Lauritzen C, Petersen GE, Pedersen J. Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expression and Purification. 2006;48(1):1–13. - PubMed
-
- Waugh DS. Making the most of affinity tags. Trends in Biotechnology. 2005;23(6):316–320. - PubMed
-
- Walls D, Loughran ST. Tagging recombinant proteins to enhance solubility and aid purification. Methods in Molecular Biology. 2011;681:151–175. - PubMed
-
- Lichty JJ, Malecki JL, Agnew HD, Michelson-Horowitz DJ, Tan S. Comparison of affinity tags for protein purification. Protein Expression and Purification. 2005;41(1):98–105. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

