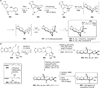
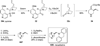
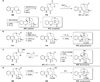
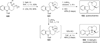
Chemistry of bridged lactams and related heterocycles
- PMID: 24490625
- PMCID: PMC4155605
- DOI: 10.1021/cr4000144
Chemistry of bridged lactams and related heterocycles
Figures

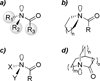
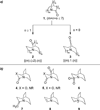



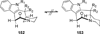
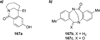







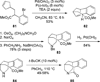

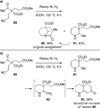

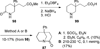
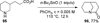







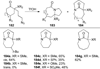
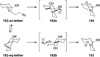
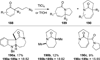
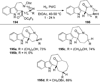









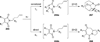
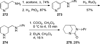
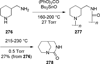
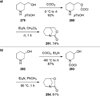











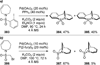
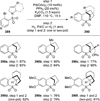


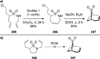
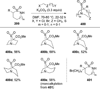






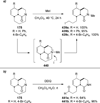
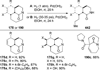
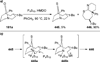


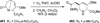




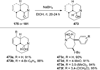
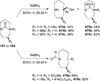
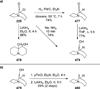

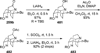















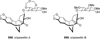
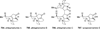
Similar articles
-
Synthetic studies on pseudo-dimeric Lycopodium alkaloids: total synthesis of complanadine B.Angew Chem Int Ed Engl. 2013 Feb 4;52(6):1726-30. doi: 10.1002/anie.201208571. Epub 2013 Jan 10. Angew Chem Int Ed Engl. 2013. PMID: 23307758 Free PMC article. No abstract available.
-
Formation of medium-sized nitrogen heterocycles from gamma-silyloxy-gamma-lactams.J Org Chem. 2009 Sep 18;74(18):6915-23. doi: 10.1021/jo900869k. J Org Chem. 2009. PMID: 19678646
-
Biogenetically inspired total syntheses of Lycopodium alkaloids, (+)-flabellidine and (-)-lycodine.J Am Chem Soc. 2014 Aug 20;136(33):11618-21. doi: 10.1021/ja507016g. Epub 2014 Aug 11. J Am Chem Soc. 2014. PMID: 25103992
-
Lycopodium alkaloids: isolation and asymmetric synthesis.Top Curr Chem. 2012;309:1-31. doi: 10.1007/128_2011_126. Top Curr Chem. 2012. PMID: 21452079 Review.
-
Construction of heterocyclic structures by trivalent cerium salts promoted bond forming reactions.Chem Soc Rev. 2014 Feb 7;43(3):779-91. doi: 10.1039/c3cs60220f. Chem Soc Rev. 2014. PMID: 24217370 Review.
Cited by
-
Synthesis of benzannelated sultams by intramolecular Pd-catalyzed arylation of tertiary sulfonamides.Beilstein J Org Chem. 2017 Sep 12;13:1932-1939. doi: 10.3762/bjoc.13.187. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 29062411 Free PMC article.
-
Radical aryl migration enables diversity-oriented synthesis of structurally diverse medium/macro- or bridged-rings.Nat Commun. 2016 Dec 22;7:13852. doi: 10.1038/ncomms13852. Nat Commun. 2016. PMID: 28004746 Free PMC article.
-
Testing the limits of radical-anionic CH-amination: a 10-million-fold decrease in basicity opens a new path to hydroxyisoindolines via a mixed C-N/C-O-forming cascade.Chem Sci. 2020 Feb 21;11(25):6539-6555. doi: 10.1039/c9sc06511c. Chem Sci. 2020. PMID: 34094120 Free PMC article.
-
Intramolecular Csp3-H/C-C bond amination of alkyl azides for the selective synthesis of cyclic imines and tertiary amines.Chem Sci. 2020 Apr 7;11(17):4482-4487. doi: 10.1039/c9sc05522c. Chem Sci. 2020. PMID: 34122906 Free PMC article.
-
Selective Conversion of Unactivated C-N Amide Bond to C-C bond via Steric and Electronic Resonance Destabilization.Org Lett. 2022 Sep 16;24(36):6525-6530. doi: 10.1021/acs.orglett.2c02420. Epub 2022 Sep 6. Org Lett. 2022. PMID: 36067532 Free PMC article.
References
-
- Greenberg A, Breneman CM, Liebman JF, editors. The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science. Wiley; 2000.
-
- Pauling L. The Nature of the Chemical Bond. London: Oxford University Press; 1940.
-
- Chalupsky J, Vondrasek J, Spirko V. J. Phys. Chem. A. 2008;112:693. - PubMed
-
- Mannfors BE, Mirkin NG, Palmo K, Krimm S. J. Phys. Chem. A. 2003;107:1825.
-
- Shin SBY, Yoo B, Todaro LJ, Kirshenbaum K. J. Am. Chem. Soc. 2007;129:3218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

