Decreased plasma levels of soluble CD18 link leukocyte infiltration with disease activity in spondyloarthritis
- PMID: 24490631
- PMCID: PMC3978678
- DOI: 10.1186/ar4471
Decreased plasma levels of soluble CD18 link leukocyte infiltration with disease activity in spondyloarthritis
Abstract
Introduction: Spondyloarthritis (SpA) comprises a group of diseases often associated with HLA-B27 and characterized by inflammation of the entheses and joints of the axial skeleton. The inflammatory process in SpA is presumably driven by innate immune cells but is still poorly understood. Thus, new tools for monitoring and treating inflammation are needed. The family of CD18 integrins is pivotal in guiding leukocytes to sites of inflammation, and CD18 hypomorphic mice develop a disease resembling SpA. Previously, we demonstrated that altered soluble CD18 (sCD18) complexes in the blood and synovial fluid of patients with arthritis have anti-inflammatory functions. Here, we study the mechanisms for these alterations and their association with SpA disease activity.
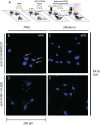
Methods: Plasma levels of sCD18 in a study population with 84 patients with SpA and matched healthy controls were analyzed with a time-resolved immunoflourometric assay (TRIFMA). Binding of sCD18 to endothelial cells and fibroblast-like synoviocytes (FLSs) was studied with confocal microscopy. Shedding of CD18 from peripheral blood mononuclear cells (PBMCs) was studied with flow cytometry and TRIFMA.
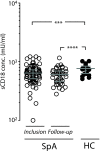
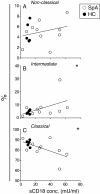
Results: Plasma levels of sCD18 were decreased in patients with SpA compared with healthy volunteers (P <0.001), and the lowest levels were in the HLA-B27-positive subgroup (P <0.05). In a multiple regression model, the sCD18 levels exhibited an inverse correlation with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (P <0.05), the level of morning stiffness (P <0.05), the Bath Ankylosing Spondilitis Metrology Index (P <0.05), the physician global assessment score (P <0.01), and the sacroiliac magnetic resonance imaging activity score (P <0.05). The mechanisms for these changes could be simulated in vitro. First, sCD18 in plasma adhered to inflammation-induced intercellular adhesion molecule 1 (ICAM-1) on endothelial cells and FLS, indicating increased consumption. Second, CD18 shedding from SpA PBMCs correlated inversely with the BASDAI (P <0.05), suggesting insufficient generation. CD18 was shed primarily from intermediate CD14⁺⁺ CD16⁺ monocytes, supporting the view that alterations in innate immunity can regulate the inflammatory processes in SpA.
Conclusions: Taken together, the failure of patients with SpA to maintain adequate sCD18 levels may reflect insufficient CD18 shedding from monocytes to counterbalance the capture of sCD18 complexes to inflammation-induced ICAM-1. This could increase the availability of ICAM-1 molecules on the endothelium and in the synovium, facilitating leukocyte migration to the entheses and joints and aggregating disease activity.
Figures

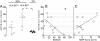


Similar articles
-
Changes in Soluble CD18 in Murine Autoimmune Arthritis and Rheumatoid Arthritis Reflect Disease Establishment and Treatment Response.PLoS One. 2016 Feb 5;11(2):e0148486. doi: 10.1371/journal.pone.0148486. eCollection 2016. PLoS One. 2016. PMID: 26849368 Free PMC article. Clinical Trial.
-
Decreased monocyte shedding of the migration inhibitor soluble CD18 in alcoholic hepatitis.Clin Transl Gastroenterol. 2018 Jun 15;9(6):160. doi: 10.1038/s41424-018-0022-7. Clin Transl Gastroenterol. 2018. PMID: 29904132 Free PMC article.
-
Large soluble CD18 complexes with exclusive ICAM-1-binding properties are shed during immune cell migration in inflammation.J Transl Autoimmun. 2025 Jan 5;10:100266. doi: 10.1016/j.jtauto.2025.100266. eCollection 2025 Jun. J Transl Autoimmun. 2025. PMID: 39867459 Free PMC article.
-
Advances in the treatment of uveitis in patients with spondyloarthritis - is it the time for biologic therapy?Rom J Ophthalmol. 2018 Apr-Jun;62(2):114-122. Rom J Ophthalmol. 2018. PMID: 30206554 Free PMC article. Review.
-
Monocytes and Macrophages in Spondyloarthritis: Functional Roles and Effects of Current Therapies.Cells. 2022 Feb 2;11(3):515. doi: 10.3390/cells11030515. Cells. 2022. PMID: 35159323 Free PMC article. Review.
Cited by
-
Toll-like receptor 2 and 4 induced interleukin-19 dampens immune reactions and associates inversely with spondyloarthritis disease activity.Clin Exp Immunol. 2015 May;180(2):233-42. doi: 10.1111/cei.12577. Clin Exp Immunol. 2015. PMID: 25639337 Free PMC article. Clinical Trial.
-
The interleukin-20 receptor axis in early rheumatoid arthritis: novel links between disease-associated autoantibodies and radiographic progression.Arthritis Res Ther. 2016 Mar 11;18:61. doi: 10.1186/s13075-016-0964-7. Arthritis Res Ther. 2016. PMID: 26968800 Free PMC article. Clinical Trial.
-
Changes in Soluble CD18 in Murine Autoimmune Arthritis and Rheumatoid Arthritis Reflect Disease Establishment and Treatment Response.PLoS One. 2016 Feb 5;11(2):e0148486. doi: 10.1371/journal.pone.0148486. eCollection 2016. PLoS One. 2016. PMID: 26849368 Free PMC article. Clinical Trial.
-
Altered levels of soluble CD18 may associate immune mechanisms with outcome in sepsis.Clin Exp Immunol. 2017 Nov;190(2):258-267. doi: 10.1111/cei.13016. Epub 2017 Aug 7. Clin Exp Immunol. 2017. PMID: 28714582 Free PMC article.
-
Structural Immunology of Complement Receptors 3 and 4.Front Immunol. 2018 Nov 26;9:2716. doi: 10.3389/fimmu.2018.02716. eCollection 2018. Front Immunol. 2018. PMID: 30534123 Free PMC article. Review.
References
-
- Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;16:2127–2137. - PubMed
-
- Braun J, van den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E, Dagfinrud H, Dijkmans B, Dougados M, Emery P, Geher P, Hammoudeh M, Inman RD, Jongkees M, Khan MA, Kiltz U, Kvien T, Leirisalo-Repo M, Maksymowych WP, Olivieri I, Pavelka K, Sieper J, Stanislawska-Biernat E, Wendling D, Ozgocmen S, van Drogen C, van Royen B, van der Heijde D. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011;16:896–904. - PMC - PubMed
-
- Maksymowych WP. Biomarkers in spondyloarthritis. Curr Rheumatol Rep. 2010;16:318–324. - PubMed
-
- Ambarus C, Yeremenko N, Tak PP, Baeten D. Pathogenesis of spondyloarthritis: autoimmune or autoinflammatory? Curr Opin Rheumatol. 2012;16:351–358. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

