A retrospective comparative exploratory study on two methylentetrahydrofolate reductase (MTHFR) polymorphisms in esophagogastric cancer: the A1298C MTHFR polymorphism is an independent prognostic factor only in neoadjuvantly treated gastric cancer patients
- PMID: 24490800
- PMCID: PMC3922603
- DOI: 10.1186/1471-2407-14-58
A retrospective comparative exploratory study on two methylentetrahydrofolate reductase (MTHFR) polymorphisms in esophagogastric cancer: the A1298C MTHFR polymorphism is an independent prognostic factor only in neoadjuvantly treated gastric cancer patients
Abstract
Background: Methylentetrahydrofolate reductase (MTHFR) plays a major role in folate metabolism and consequently could be an important factor for the efficacy of a treatment with 5-fluorouracil. Our aim was to evaluate the prognostic and predictive value of two well characterized constitutional MTHFR gene polymorphisms for primarily resected and neoadjuvantly treated esophagogastric adenocarcinomas.
Methods: 569 patients from two centers were analyzed (gastric cancer: 218, carcinoma of the esophagogastric junction (AEG II, III): 208 and esophagus (AEG I): 143). 369 patients received neoadjuvant chemotherapy followed by surgery, 200 patients were resected without preoperative treatment. The MTHFR C677T and A1298C polymorphisms were determined in DNA from peripheral blood lymphozytes. Associations with prognosis, response and clinicopathological factors were analyzed retrospectively within a prospective database (chi-square, log-rank, cox regression).
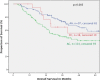
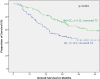
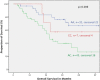
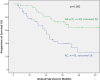
Results: Only the MTHFR A1298C polymorphisms had prognostic relevance in neoadjuvantly treated patients but it was not a predictor for response to neoadjuvant chemotherapy. The AC genotype of the MTHFR A1298C polymorphisms was significantly associated with worse outcome (p = 0.02, HR 1.47 (1.06-2.04). If neoadjuvantly treated patients were analyzed based on their tumor localization, the AC genotype of the MTHFR A1298C polymorphisms was a significant negative prognostic factor in patients with gastric cancer according to UICC 6th edition (gastric cancer including AEG type II, III: HR 2.0, 95% CI 1.3-2.0, p = 0.001) and 7th edition (gastric cancer without AEG II, III: HR 2.8, 95% CI 1.5-5.7, p = 0.003), not for AEG I. For both definitions of gastric cancer the AC genotype was confirmed as an independent negative prognostic factor in cox regression analysis. In primarily resected patients neither the MTHFR A1298C nor the MTHFR C677T polymorphisms had prognostic impact.
Conclusions: The MTHFR A1298C polymorphisms was an independent prognostic factor in patients with neoadjuvantly treated gastric adenocarcinomas (according to both UICC 6th or 7th definitions for gastric cancer) but not in AEG I nor in primarily resected patients, which confirms the impact of this enzyme on chemotherapy associated outcome.
Figures

References
-
- Cunningham D, Allum W, Stenning S, Thompson J, Van de Velde C, Nicolson M, Scarffe J, Lofts F, Falk S, Iveson T, Smith D, Langley R, Verma M, Weeden S, Chua Y. MAGIC trial participants: perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. - DOI - PubMed
-
- Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–1721. doi: 10.1200/JCO.2010.33.0597. - DOI - PubMed
-
- van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A, Group C. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. - DOI - PubMed
-
- Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V, Group AG-IT. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–692. doi: 10.1016/S1470-2045(11)70142-5. - DOI - PubMed
-
- Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J, Friess H, Hofler H. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011;253(5):934–939. doi: 10.1097/SLA.0b013e318216f449. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

