Biochemical and structural analysis of the hyperpolarization-activated K(+) channel MVP
- PMID: 24490868
- PMCID: PMC3985891
- DOI: 10.1021/bi4014243
Biochemical and structural analysis of the hyperpolarization-activated K(+) channel MVP
Abstract
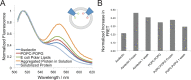
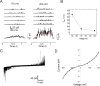
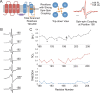
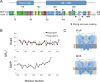
In contrast to the majority of voltage-gated ion channels, hyperpolarization-activated channels remain closed at depolarizing potentials and are activated at hyperpolarizing potentials. The basis for this reverse polarity is thought to be a result of differences in the way the voltage-sensing domain (VSD) couples to the pore domain. In the absence of structural data, the molecular mechanism of this reverse polarity coupling remains poorly characterized. Here we report the characterization of the structure and local dynamics of the closed activation gate (lower S6 region) of MVP, a hyperpolarization-activated potassium channel from Methanococcus jannaschii, by electron paramagnetic resonance (EPR) spectroscopy. We show that a codon-optimized version of MVP has high expression levels in Escherichia coli, is purified as a stable tetramer, and exhibits expected voltage-dependent activity when reconstituted in liposomes. EPR analysis of the mid to lower S6 region revealed positions exhibiting strong spin-spin coupling, indicating that the activation gate of MVP is closed at 0 mV. A comparison of local environmental parameters along the activation gate for MVP and KcsA indicates that MVP adopts a different closed conformation. These structural details set the stage for future evaluations of reverse electromechanical coupling in MVP.
Figures


Similar articles
-
Molecular mechanism of a potassium channel gating through activation gate-selectivity filter coupling.Nat Commun. 2019 Nov 26;10(1):5366. doi: 10.1038/s41467-019-13227-w. Nat Commun. 2019. PMID: 31772184 Free PMC article.
-
Hysteresis of KcsA potassium channel's activation- deactivation gating is caused by structural changes at the channel's selectivity filter.Proc Natl Acad Sci U S A. 2017 Mar 21;114(12):3234-3239. doi: 10.1073/pnas.1618101114. Epub 2017 Mar 6. Proc Natl Acad Sci U S A. 2017. PMID: 28265056 Free PMC article.
-
Voltage-dependent gating of hyperpolarization-activated, cyclic nucleotide-gated pacemaker channels: molecular coupling between the S4-S5 and C-linkers.J Biol Chem. 2004 Apr 2;279(14):13859-65. doi: 10.1074/jbc.M313704200. Epub 2004 Jan 15. J Biol Chem. 2004. PMID: 14726518
-
Understanding the conformational motions of RCK gating rings.J Gen Physiol. 2017 Apr 3;149(4):431-441. doi: 10.1085/jgp.201611726. Epub 2017 Feb 28. J Gen Physiol. 2017. PMID: 28246116 Free PMC article. Review.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
Combining in Vitro Folding with Cell Free Protein Synthesis for Membrane Protein Expression.Biochemistry. 2016 Aug 2;55(30):4212-9. doi: 10.1021/acs.biochem.6b00488. Epub 2016 Jul 21. Biochemistry. 2016. PMID: 27384110 Free PMC article.
-
Cholesterol-Dependent Gating Effects on Ion Channels.Adv Exp Med Biol. 2019;1115:167-190. doi: 10.1007/978-3-030-04278-3_8. Adv Exp Med Biol. 2019. PMID: 30649760 Free PMC article. Review.
-
Gating interaction maps reveal a noncanonical electromechanical coupling mode in the Shaker K+ channel.Nat Struct Mol Biol. 2018 Apr;25(4):320-326. doi: 10.1038/s41594-018-0047-3. Epub 2018 Mar 26. Nat Struct Mol Biol. 2018. PMID: 29581567 Free PMC article.
-
Exploring Flexibility and Folding Patterns Throughout Time in Voltage Sensors.J Mol Evol. 2023 Dec;91(6):819-836. doi: 10.1007/s00239-023-10140-1. Epub 2023 Nov 13. J Mol Evol. 2023. PMID: 37955698
-
Functional hemichannels formed by human connexin 26 expressed in bacteria.Biosci Rep. 2015 Mar 18;35(2):e00177. doi: 10.1042/BSR20140089. Biosci Rep. 2015. PMID: 25585383 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

