Nitrogen-containing bisphosphonates inhibit RANKL- and M-CSF-induced osteoclast formation through the inhibition of ERK1/2 and Akt activation
- PMID: 24490900
- PMCID: PMC3996180
- DOI: 10.1186/1423-0127-21-10
Nitrogen-containing bisphosphonates inhibit RANKL- and M-CSF-induced osteoclast formation through the inhibition of ERK1/2 and Akt activation
Abstract
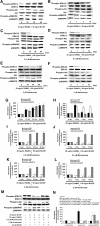
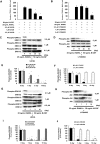
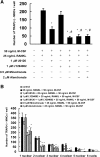
Background: Bisphosphonates are an important class of antiresorptive drugs used in the treatment of metabolic bone diseases. Recent studies have shown that nitrogen-containing bisphosphonates induced apoptosis in rabbit osteoclasts and prevented prenylated small GTPase. However, whether bisphosphonates inhibit osteoclast formation has not been determined. In the present study, we investigated the inhibitory effect of minodronate and alendronate on the osteoclast formation and clarified the mechanism involved in a mouse macrophage-like cell lines C7 and RAW264.7.
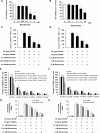
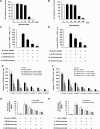
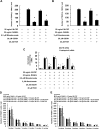
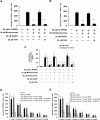
Results: It was found that minodronate and alendronate inhibited the osteoclast formation of C7 cells induced by receptor activator of NF-κB ligand and macrophage colony stimulating factor, which are inhibited by the suppression of geranylgeranyl pyrophosphate (GGPP) biosynthesis. It was also found that minodronate and alendronate inhibited the osteoclast formation of RAW264.7 cells induced by receptor activator of NF-κB ligand. Furthermore, minodronate and alendornate decreased phosphorylated extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt; similarly, U0126, a mitogen protein kinase kinase 1/2 (MEK1/2) inhibitor, and LY294002, a phosphatidylinositol 3-kinase (PI3K) inhibitor, inhibited osteoclast formation.
Conclusions: This indicates that minodronate and alendronate inhibit GGPP biosynthesis in the mevalonate pathway and then signal transduction in the MEK/ERK and PI3K/Akt pathways, thereby inhibiting osteoclast formation. These results suggest a novel effect of bisphosphonates that could be effective in the treatment of bone metabolic diseases, such as osteoporosis.
Figures
Similar articles
-
Nitrogen-containing bisphosphonate, YM529/ONO-5920 (a novel minodronic acid), inhibits RANKL expression in a cultured bone marrow stromal cell line ST2.Biochem Biophys Res Commun. 2005 Mar 4;328(1):91-7. doi: 10.1016/j.bbrc.2004.12.145. Biochem Biophys Res Commun. 2005. PMID: 15670755
-
RANKL increases the level of Mcl-1 in osteoclasts and reduces bisphosphonate-induced osteoclast apoptosis in vitro.Arthritis Res Ther. 2009;11(2):R58. doi: 10.1186/ar2681. Epub 2009 Apr 30. Arthritis Res Ther. 2009. PMID: 19405951 Free PMC article.
-
Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-κB ligand–induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+–nuclear factor of activated T-cells cytoplasmic 1 signaling pathways.J Bone Miner Res. 2012 Jun;27(6):1298-1308. doi: 10.1002/jbmr.1576. J Bone Miner Res. 2012. PMID: 22337253
-
Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras.J Bone Miner Res. 1998 Apr;13(4):581-9. doi: 10.1359/jbmr.1998.13.4.581. J Bone Miner Res. 1998. PMID: 9556058
-
Bisphosphonates: from the laboratory to the clinic and back again.Bone. 1999 Jul;25(1):97-106. doi: 10.1016/s8756-3282(99)00116-7. Bone. 1999. PMID: 10423031 Review.
Cited by
-
Increased trabecular bone and improved biomechanics in an osteocalcin-null rat model created by CRISPR/Cas9 technology.Dis Model Mech. 2016 Oct 1;9(10):1169-1179. doi: 10.1242/dmm.025247. Epub 2016 Jul 28. Dis Model Mech. 2016. PMID: 27483347 Free PMC article.
-
Dihydromyricetin Protects against Bone Loss in Ovariectomized Mice by Suppressing Osteoclast Activity.Front Pharmacol. 2017 Dec 19;8:928. doi: 10.3389/fphar.2017.00928. eCollection 2017. Front Pharmacol. 2017. PMID: 29311931 Free PMC article.
-
Molecular Mechanisms Responsible for the Rescue Effects of Pamidronate on Muscle Atrophy in Pediatric Burn Patients.Front Endocrinol (Lausanne). 2019 Aug 7;10:543. doi: 10.3389/fendo.2019.00543. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31447786 Free PMC article.
-
Effects of switching weekly alendronate or risedronate to monthly minodronate in patients with rheumatoid arthritis: a 12-month prospective study.Osteoporos Int. 2016 Jan;27(1):351-9. doi: 10.1007/s00198-015-3369-6. Osteoporos Int. 2016. PMID: 26475289
-
The Potential of Enamel Matrix Derivative in Countering Bisphosphonate-Induced Effects in Osteoblasts.Life (Basel). 2024 Aug 29;14(9):1088. doi: 10.3390/life14091088. Life (Basel). 2024. PMID: 39337872 Free PMC article.
References
-
- Roodman GD. Advances in bone biology: the osteoclast. Endocr Rev. 1996;17:308–332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

