PrtR homeostasis contributes to Pseudomonas aeruginosa pathogenesis and resistance against ciprofloxacin
- PMID: 24491574
- PMCID: PMC3993409
- DOI: 10.1128/IAI.01388-13
PrtR homeostasis contributes to Pseudomonas aeruginosa pathogenesis and resistance against ciprofloxacin
Abstract
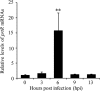
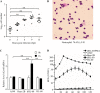
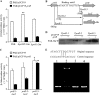
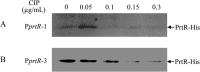
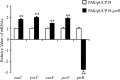
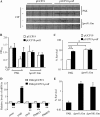
Pseudomonas aeruginosa is an opportunistic pathogen that causes acute and chronic infections in humans. Pyocins are bacteriocins produced by P. aeruginosa that are usually released through lysis of the producer strains. Expression of pyocin genes is negatively regulated by PrtR, which gets cleaved under SOS response, leading to upregulation of pyocin synthetic genes. Previously, we demonstrated that PrtR is required for the expression of type III secretion system (T3SS), which is an important virulence component of P. aeruginosa. In this study, we demonstrate that mutation in prtR results in reduced bacterial colonization in a mouse acute pneumonia model. Examination of bacterial and host cells in the bronchoalveolar lavage fluids from infected mice revealed that expression of PrtR is induced by reactive oxygen species (ROS) released by neutrophils. We further demonstrate that treatment with hydrogen peroxide or ciprofloxacin, known to induce the SOS response and pyocin production, resulted in an elevated PrtR mRNA level. Overexpression of PrtR by a tac promoter repressed the endogenous prtR promoter activity, and electrophoretic mobility shift assay revealed that PrtR binds to its own promoter, suggesting an autorepressive mechanism of regulation. A high level of PrtR expressed from a plasmid resulted in increased T3SS gene expression during infection and higher resistance against ciprofloxacin. Overall, our results suggest that the autorepression of PrtR contributes to the maintenance of a relatively stable level of PrtR, which is permissive to T3SS gene expression in the presence of ROS while increasing bacterial tolerance to stresses, such as ciprofloxacin, by limiting pyocin production.
Figures

Similar articles
-
Pyocins and Beyond: Exploring the World of Bacteriocins in Pseudomonas aeruginosa.Probiotics Antimicrob Proteins. 2025 Feb;17(1):240-252. doi: 10.1007/s12602-024-10322-3. Epub 2024 Jul 18. Probiotics Antimicrob Proteins. 2025. PMID: 39023701 Review.
-
Fis Contributes to Resistance of Pseudomonas aeruginosa to Ciprofloxacin by Regulating Pyocin Synthesis.J Bacteriol. 2020 May 11;202(11):e00064-20. doi: 10.1128/JB.00064-20. Print 2020 May 11. J Bacteriol. 2020. PMID: 32205461 Free PMC article.
-
Biological cost of pyocin production during the SOS response in Pseudomonas aeruginosa.J Bacteriol. 2014 Sep;196(18):3351-9. doi: 10.1128/JB.01889-14. Epub 2014 Jul 14. J Bacteriol. 2014. PMID: 25022851 Free PMC article.
-
Pseudomonas aeruginosa Oligoribonuclease Contributes to Tolerance to Ciprofloxacin by Regulating Pyocin Biosynthesis.Antimicrob Agents Chemother. 2017 Feb 23;61(3):e02256-16. doi: 10.1128/AAC.02256-16. Print 2017 Mar. Antimicrob Agents Chemother. 2017. PMID: 28052848 Free PMC article.
-
Mechanisms of ciprofloxacin resistance in Pseudomonas aeruginosa: new approaches to an old problem.J Med Microbiol. 2019 Jan;68(1):1-10. doi: 10.1099/jmm.0.000873. J Med Microbiol. 2019. PMID: 30605076 Review.
Cited by
-
NupR Responding to Multiple Signals Is a Nucleoside Permease Regulator in Bacillus thuringiensis BMB171.Microbiol Spectr. 2022 Aug 31;10(4):e0154322. doi: 10.1128/spectrum.01543-22. Epub 2022 Jul 7. Microbiol Spectr. 2022. PMID: 35862946 Free PMC article.
-
Pyocins and Beyond: Exploring the World of Bacteriocins in Pseudomonas aeruginosa.Probiotics Antimicrob Proteins. 2025 Feb;17(1):240-252. doi: 10.1007/s12602-024-10322-3. Epub 2024 Jul 18. Probiotics Antimicrob Proteins. 2025. PMID: 39023701 Review.
-
PvrA is a novel regulator that contributes to Pseudomonas aeruginosa pathogenesis by controlling bacterial utilization of long chain fatty acids.Nucleic Acids Res. 2020 Jun 19;48(11):5967-5985. doi: 10.1093/nar/gkaa377. Nucleic Acids Res. 2020. PMID: 32406921 Free PMC article.
-
Targeting Bacterial Gyrase with Cystobactamid, Fluoroquinolone, and Aminocoumarin Antibiotics Induces Distinct Molecular Signatures in Pseudomonas aeruginosa.mSystems. 2021 Aug 31;6(4):e0061021. doi: 10.1128/mSystems.00610-21. Epub 2021 Jul 13. mSystems. 2021. PMID: 34254824 Free PMC article.
-
Genomic Insights into the Pathogenicity of Hypervirulent Aeromonas hydrophila Strain D4 Isolated from Diseased Blunt Snout Bream with the Epidemic Sequence Type 251 Clones.Pathogens. 2025 Jun 6;14(6):570. doi: 10.3390/pathogens14060570. Pathogens. 2025. PMID: 40559578 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

