DNA vaccines encoding altered peptide ligands for SSX2 enhance epitope-specific CD8+ T-cell immune responses
- PMID: 24492013
- PMCID: PMC4153342
- DOI: 10.1016/j.vaccine.2014.01.048
DNA vaccines encoding altered peptide ligands for SSX2 enhance epitope-specific CD8+ T-cell immune responses
Abstract
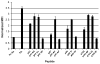
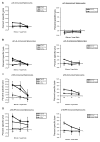
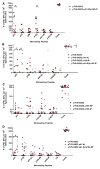
Plasmid DNA serves as a simple and easily modifiable form of antigen delivery for vaccines. The USDA approval of DNA vaccines for several non-human diseases underscores the potential of this type of antigen delivery method as a cost-effective approach for the treatment or prevention of human diseases, including cancer. However, while DNA vaccines have demonstrated safety and immunological effect in early phase clinical trials, they have not consistently elicited robust anti-tumor responses. Hence many recent efforts have sought to increase the immunological efficacy of DNA vaccines, and we have specifically evaluated several target antigens encoded by DNA vaccine as treatments for human prostate cancer. In particular, we have focused on SSX2 as one potential target antigen, given its frequent expression in metastatic prostate cancer. We have previously identified two peptides, p41-49 and p103-111, as HLA-A2-restricted SSX2-specific epitopes. In the present study we sought to determine whether the efficacy of a DNA vaccine could be enhanced by an altered peptide ligand (APL) strategy wherein modifications were made to anchor residues of these epitopes to enhance or ablate their binding to HLA-A2. A DNA vaccine encoding APL modified to increase epitope binding elicited robust peptide-specific CD8+ T cells producing Th1 cytokines specific for each epitope. Ablation of one epitope in a DNA vaccine did not enhance immune responses to the other epitope. These results demonstrate that APL encoded by a DNA vaccine can be used to elicit increased numbers of antigen-specific T cells specific for multiple epitopes simultaneously, and suggest this could be a general approach to improve the immunogenicity of DNA vaccines encoding tumor antigens.
Keywords: APL; CTA; DNA vaccine; Prostate cancer; SSX.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

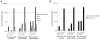
Similar articles
-
Vaccines targeting the cancer-testis antigen SSX-2 elicit HLA-A2 epitope-specific cytolytic T cells.J Immunother. 2011 Oct;34(8):569-80. doi: 10.1097/CJI.0b013e31822b5b1d. J Immunother. 2011. PMID: 21904219 Free PMC article.
-
A peptide epitope derived from the cancer testis antigen HOM-MEL-40/SSX2 capable of inducing CD4⁺ and CD8⁺ T-cell as well as B-cell responses.Cancer Immunol Immunother. 2011 Sep;60(9):1333-46. doi: 10.1007/s00262-011-1030-6. Epub 2011 Jun 1. Cancer Immunol Immunother. 2011. PMID: 21630107 Free PMC article.
-
HLA-A2-restricted T-cell epitopes specific for prostatic acid phosphatase.Cancer Immunol Immunother. 2010 Jun;59(6):943-53. doi: 10.1007/s00262-010-0820-6. Epub 2010 Feb 6. Cancer Immunol Immunother. 2010. PMID: 20140431 Free PMC article.
-
The use of HLA class I tetramers to design a vaccination strategy for melanoma patients.Immunol Rev. 2002 Oct;188:155-63. doi: 10.1034/j.1600-065x.2002.18814.x. Immunol Rev. 2002. PMID: 12445289 Review.
-
Design of multi-epitope, analogue-based cancer vaccines.Expert Opin Biol Ther. 2003 Sep;3(6):985-93. doi: 10.1517/14712598.3.6.985. Expert Opin Biol Ther. 2003. PMID: 12943457 Review.
Cited by
-
PD-1 and LAG-3 blockade improve anti-tumor vaccine efficacy.Oncoimmunology. 2021 Apr 21;10(1):1912892. doi: 10.1080/2162402X.2021.1912892. Oncoimmunology. 2021. PMID: 33996265 Free PMC article.
-
Trial watch: DNA-based vaccines for oncological indications.Oncoimmunology. 2017 Nov 20;6(12):e1398878. doi: 10.1080/2162402X.2017.1398878. eCollection 2017. Oncoimmunology. 2017. PMID: 29209575 Free PMC article. Review.
-
Vaccination with High-Affinity Epitopes Impairs Antitumor Efficacy by Increasing PD-1 Expression on CD8+ T Cells.Cancer Immunol Res. 2017 Aug;5(8):630-641. doi: 10.1158/2326-6066.CIR-16-0374. Epub 2017 Jun 20. Cancer Immunol Res. 2017. PMID: 28634215 Free PMC article.
-
B lymphocytes as direct antigen-presenting cells for anti-tumor DNA vaccines.Oncotarget. 2016 Oct 18;7(42):67901-67918. doi: 10.18632/oncotarget.12178. Oncotarget. 2016. PMID: 27661128 Free PMC article.
-
Immunotherapy in prostate cancer: review of the current evidence.Clin Transl Oncol. 2015 May;17(5):339-57. doi: 10.1007/s12094-014-1259-6. Epub 2014 Dec 6. Clin Transl Oncol. 2015. PMID: 25480118 Review.
References
-
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–8. - PubMed
-
- Iwasaki A, Torres CA, Ohashi PS, Robinson HL, Barber BH. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J Immunol. 1997;159:11–4. - PubMed
-
- Alonso M, Leong JA. Licensed DNA vaccines against infectious hematopoietic necrosis virus (IHNV) Recent Pat DNA Gene Seq. 2013;7:62–5. - PubMed
-
- Hall RA, Khromykh AA. West Nile virus vaccines. Expert Opin Biol Ther. 2004;4:1295–305. - PubMed
-
- Grosenbaugh DA, Leard AT, Bergman PJ, Klein MK, Meleo K, Susaneck S, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res. 2011;72:1631–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

