Vegetative and sperm cell-specific aquaporins of Arabidopsis highlight the vacuolar equipment of pollen and contribute to plant reproduction
- PMID: 24492334
- PMCID: PMC3982734
- DOI: 10.1104/pp.113.228700
Vegetative and sperm cell-specific aquaporins of Arabidopsis highlight the vacuolar equipment of pollen and contribute to plant reproduction
Abstract
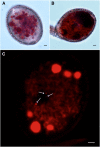
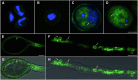
The water and nutrient status of pollen is crucial to plant reproduction. Pollen grains of Arabidopsis (Arabidopsis thaliana) contain a large vegetative cell and two smaller sperm cells. Pollen grains express AtTIP1;3 and AtTIP5;1, two members of the Tonoplast Intrinsic Protein subfamily of aquaporins. To address the spatial and temporal expression pattern of the two homologs, C-terminal fusions of AtTIP1;3 and AtTIP5;1 with green fluorescent protein and mCherry, respectively, were expressed in transgenic Arabidopsis under the control of their native promoter. Confocal laser scanning microscopy revealed that AtTIP1;3 and AtTIP5;1 are specific for the vacuoles of the vegetative and sperm cells, respectively. The tonoplast localization of AtTIP5;1 was established by reference to fluorescent protein markers for the mitochondria and vacuoles of sperm and vegetative cells and is at variance with the claim that AtTIP5;1 is localized in vegetative cell mitochondria. AtTIP1;3-green fluorescent protein and AtTIP5;1-mCherry showed concomitant expression, from first pollen mitosis up to pollen tube penetration in the ovule, thereby revealing the dynamics of vacuole morphology in maturating and germinating pollen. Transfer DNA insertion mutants for either AtTIP1;3 or AtTIP5;1 showed no apparent growth phenotype and had no significant defect in male transmission of the mutated alleles. By contrast, a double knockout displayed an abnormal rate of barren siliques, this phenotype being more pronounced under limited water or nutrient supply. The overall data indicate that vacuoles of vegetative and sperm cells functionally interact and contribute to male fertility in adverse environmental conditions.
Figures



Similar articles
-
The ER-localized aquaporin SIP2;1 is involved in pollen germination and pollen tube elongation in Arabidopsis thaliana.Plant Mol Biol. 2019 Jun;100(3):335-349. doi: 10.1007/s11103-019-00865-3. Epub 2019 Apr 8. Plant Mol Biol. 2019. PMID: 30963359
-
AtTIP1;3 and AtTIP5;1, the only highly expressed Arabidopsis pollen-specific aquaporins, transport water and urea.FEBS Lett. 2008 Dec 10;582(29):4077-82. doi: 10.1016/j.febslet.2008.11.002. Epub 2008 Nov 18. FEBS Lett. 2008. PMID: 19022253
-
Life with and without AtTIP1;1, an Arabidopsis aquaporin preferentially localized in the apposing tonoplasts of adjacent vacuoles.Plant Mol Biol. 2009 May;70(1-2):193-209. doi: 10.1007/s11103-009-9465-2. Epub 2009 Feb 20. Plant Mol Biol. 2009. PMID: 19229639
-
Overexpression of the tonoplast aquaporin AtTIP5;1 conferred tolerance to boron toxicity in Arabidopsis.J Genet Genomics. 2010 Jun;37(6):389-97, 1-2. doi: 10.1016/S1673-8527(09)60057-6. Epub 2010 Jul 1. J Genet Genomics. 2010. PMID: 20621021
-
Vacuolar trafficking in pollen tube growth and guidance.Plant Signal Behav. 2018;13(5):e1464854. doi: 10.1080/15592324.2018.1464854. Epub 2018 Jun 26. Plant Signal Behav. 2018. PMID: 29701540 Free PMC article. Review.
Cited by
-
Pollen-Specific Aquaporins NIP4;1 and NIP4;2 Are Required for Pollen Development and Pollination in Arabidopsis thaliana.Plant Cell. 2016 May;28(5):1053-77. doi: 10.1105/tpc.15.00776. Epub 2016 Apr 19. Plant Cell. 2016. PMID: 27095837 Free PMC article.
-
The ER-localized aquaporin SIP2;1 is involved in pollen germination and pollen tube elongation in Arabidopsis thaliana.Plant Mol Biol. 2019 Jun;100(3):335-349. doi: 10.1007/s11103-019-00865-3. Epub 2019 Apr 8. Plant Mol Biol. 2019. PMID: 30963359
-
Arabidopsis VAC14 Is Critical for Pollen Development through Mediating Vacuolar Organization.Plant Physiol. 2018 Aug;177(4):1529-1538. doi: 10.1104/pp.18.00495. Epub 2018 Jun 8. Plant Physiol. 2018. PMID: 29884680 Free PMC article.
-
Genetic control of generative cell shape by DUO1 in Arabidopsis.Plant Reprod. 2023 Sep;36(3):243-254. doi: 10.1007/s00497-023-00462-x. Epub 2023 Apr 6. Plant Reprod. 2023. PMID: 37022491 Free PMC article.
-
Plant and Mammal Aquaporins: Same but Different.Int J Mol Sci. 2018 Feb 8;19(2):521. doi: 10.3390/ijms19020521. Int J Mol Sci. 2018. PMID: 29419811 Free PMC article. Review.
References
-
- Alexander MP. (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44: 117–122 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

