Antifibrotic effects of noscapine through activation of prostaglandin E2 receptors and protein kinase A
- PMID: 24492608
- PMCID: PMC3953264
- DOI: 10.1074/jbc.M113.546812
Antifibrotic effects of noscapine through activation of prostaglandin E2 receptors and protein kinase A
Abstract
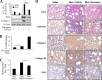
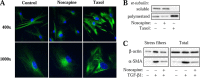
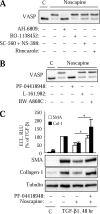
Myofibroblast differentiation is a key process in the pathogenesis of fibrotic disease. We have shown previously that differentiation of myofibroblasts is regulated by microtubule polymerization state. In this work, we examined the potential antifibrotic effects of the antitussive drug, noscapine, recently found to bind microtubules and affect microtubule dynamics. Noscapine inhibited TGF-β-induced differentiation of cultured human lung fibroblasts (HLFs). Therapeutic noscapine treatment resulted in a significant attenuation of pulmonary fibrosis in the bleomycin model of the disease. Noscapine did not affect gross microtubule content in HLFs, but inhibited TGF-β-induced stress fiber formation and activation of serum response factor without affecting Smad signaling. Furthermore, noscapine stimulated a rapid and profound activation of protein kinase A (PKA), which mediated the antifibrotic effect of noscapine in HLFs, as assessed with the PKA inhibitor, PKI. In contrast, noscapine did not activate PKA in human bronchial or alveolar epithelial cells. Finally, activation of PKA and the antifibrotic effect of noscapine in HLFs were blocked by the EP2 prostaglandin E2 receptor antagonist, PF-04418948, but not by the antagonists of EP4, prostaglandin D2, or prostacyclin receptors. Together, we demonstrate for the first time the antifibrotic effect of noscapine in vitro and in vivo, and we describe a novel mechanism of noscapine action through EP2 prostaglandin E2 receptor-mediated activation of PKA in pulmonary fibroblasts.
Keywords: Fibrosis; Microtubules; Myofibroblast; Noscapine; Prostaglandins; Protein Kinase A (PKA); SRF; Stress Fibers.
Figures


Similar articles
-
Regulation of myofibroblast differentiation and bleomycin-induced pulmonary fibrosis by adrenomedullin.Am J Physiol Lung Cell Mol Physiol. 2013 Jun 1;304(11):L757-64. doi: 10.1152/ajplung.00262.2012. Epub 2013 Apr 12. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23585227 Free PMC article.
-
Prostaglandin E2 inhibits profibrotic function of human pulmonary fibroblasts by disrupting Ca2+ signaling.Am J Physiol Lung Cell Mol Physiol. 2019 May 1;316(5):L810-L821. doi: 10.1152/ajplung.00403.2018. Epub 2019 Feb 13. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30758990 Free PMC article.
-
Control of myofibroblast differentiation by microtubule dynamics through a regulated localization of mDia2.J Biol Chem. 2013 May 31;288(22):15466-73. doi: 10.1074/jbc.M113.464461. Epub 2013 Apr 11. J Biol Chem. 2013. PMID: 23580645 Free PMC article.
-
A review on noscapine, and its impact on heme metabolism.Curr Drug Metab. 2013 Mar;14(3):351-60. doi: 10.2174/1389200211314030010. Curr Drug Metab. 2013. PMID: 22935070 Review.
-
Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages.Curr Pharm Des. 2007;13(12):1247-56. doi: 10.2174/138161207780618885. Curr Pharm Des. 2007. PMID: 17504233 Review.
Cited by
-
Megakaryoblastic leukemia-1 is required for the development of bleomycin-induced pulmonary fibrosis.Respir Res. 2015 Mar 27;16(1):45. doi: 10.1186/s12931-015-0206-6. Respir Res. 2015. PMID: 25885656 Free PMC article.
-
Sustained Smad2 Phosphorylation Is Required for Myofibroblast Transformation in Response to TGF-β.Am J Respir Cell Mol Biol. 2019 Mar;60(3):367-369. doi: 10.1165/rcmb.2018-0252LE. Am J Respir Cell Mol Biol. 2019. PMID: 30821500 Free PMC article. No abstract available.
-
Noscapine Acts as a Protease Inhibitor of In Vitro Elastase-Induced Collagen Deposition in Equine Endometrium.Int J Mol Sci. 2021 May 19;22(10):5333. doi: 10.3390/ijms22105333. Int J Mol Sci. 2021. PMID: 34069423 Free PMC article.
-
SIRT3 blocks myofibroblast differentiation and pulmonary fibrosis by preventing mitochondrial DNA damage.Am J Physiol Lung Cell Mol Physiol. 2017 Jan 1;312(1):L68-L78. doi: 10.1152/ajplung.00188.2016. Epub 2016 Nov 4. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 27815257 Free PMC article.
-
Gα12 and Gα13 proteins are required for transforming growth factor-β-induced myofibroblast differentiation.Biochem J. 2024 Dec 18;481(24):1937-1948. doi: 10.1042/BCJ20240317. Biochem J. 2024. PMID: 39621448 Free PMC article.
References
-
- Olson A. L., Swigris J. J., Lezotte D. C., Norris J. M., Wilson C. G., Brown K. K. (2007) Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am. J. Respir. Crit. Care Med. 176, 277–284 - PubMed
-
- Noble P. W., Homer R. J. (2005) Back to the future: historical perspective on the pathogenesis of idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 33, 113–120 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

