Subanesthetic ketamine treatment promotes abnormal interactions between neural subsystems and alters the properties of functional brain networks
- PMID: 24492765
- PMCID: PMC4023152
- DOI: 10.1038/npp.2014.26
Subanesthetic ketamine treatment promotes abnormal interactions between neural subsystems and alters the properties of functional brain networks
Abstract
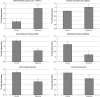
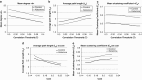
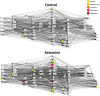
Acute treatment with subanesthetic ketamine, a non-competitive N-methyl-D-aspartic acid (NMDA) receptor antagonist, is widely utilized as a translational model for schizophrenia. However, how acute NMDA receptor blockade impacts on brain functioning at a systems level, to elicit translationally relevant symptomatology and behavioral deficits, has not yet been determined. Here, for the first time, we apply established and recently validated topological measures from network science to brain imaging data gained from ketamine-treated mice to elucidate how acute NMDA receptor blockade impacts on the properties of functional brain networks. We show that the effects of acute ketamine treatment on the global properties of these networks are divergent from those widely reported in schizophrenia. Where acute NMDA receptor blockade promotes hyperconnectivity in functional brain networks, pronounced dysconnectivity is found in schizophrenia. We also show that acute ketamine treatment increases the connectivity and importance of prefrontal and thalamic brain regions in brain networks, a finding also divergent to alterations seen in schizophrenia. In addition, we characterize how ketamine impacts on bipartite functional interactions between neural subsystems. A key feature includes the enhancement of prefrontal cortex (PFC)-neuromodulatory subsystem connectivity in ketamine-treated animals, a finding consistent with the known effects of ketamine on PFC neurotransmitter levels. Overall, our data suggest that, at a systems level, acute ketamine-induced alterations in brain network connectivity do not parallel those seen in chronic schizophrenia. Hence, the mechanisms through which acute ketamine treatment induces translationally relevant symptomatology may differ from those in chronic schizophrenia. Future effort should therefore be dedicated to resolve the conflicting observations between this putative translational model and schizophrenia.
Figures

References
-
- Amargos-Bosch M, Lopez-Gill X, Artigas F, Adell A. Clozapine and olanzapine, but not haloperidol, suppress serotonin efflux in the medial prefrontal cortex elicited by phencyclidine and ketamine. Int J Neuropsychopharmacol. 2006;9:565–573. - PubMed
-
- Bakshi VP, Geyer MA. Phencyclidine induced deficits in prepulse inhibition of startle are blocked by prazosin, an alpha-1 noradrenergic antagonist. J Pharmacol Exp Ther. 1997;283:666–674. - PubMed
-
- Benetti S, Mechelli A, Picchioni M, Broome M, Williams S, McGuire P. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132:2426–2436. - PubMed
-
- Bifone A, Gozzi A, Schwarz AJ. Functional connectivity in the rat brain: a complex network approach. Magn Reson Imaging. 2010;28:1200–1209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

