Innate and adaptive type 2 immune cell responses in genetically controlled resistance to intestinal helminth infection
- PMID: 24492801
- PMCID: PMC4038150
- DOI: 10.1038/icb.2013.109
Innate and adaptive type 2 immune cell responses in genetically controlled resistance to intestinal helminth infection
Abstract
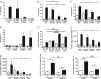
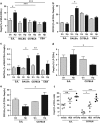
The nematode Heligmosomoides polygyrus is an excellent model for intestinal helminth parasitism. Infection in mice persists for varying lengths of time in different inbred strains, with CBA and C57BL/6 mice being fully susceptible, BALB/c partially so and SJL able to expel worms within 2-3 weeks of infection. We find that resistance correlates not only with the adaptive Th2 response, including IL-10 but with activation of innate lymphoid cell and macrophage populations. In addition, the titer and specificity range of the serum antibody response is maximal in resistant mice. In susceptible strains, Th2 responses were found to be counterbalanced by IFN-γ-producing CD4(+) and CD8(+) cells, but these are not solely responsible for susceptibility as mice deficient in either CD8(+) T cells or IFN-γ remain unable to expel the parasites. Foxp3(+) Treg numbers were comparable in all strains, but in the most resistant SJL strain, this population does not upregulate CD103 in infection, and in the lamina propria the frequency of Foxp3(+)CD103(+) T cells is significantly lower than in susceptible mice. The more resistant SJL and BALB/c mice develop macrophage-rich IL-4Rα-dependent Type 2 granulomas around intestinal sites of larval invasion, and expression of alternative activation markers Arginase-1, Ch3L3 (Ym1) and RELM-α within the intestine and the peritoneal lavage was also strongly correlated with helminth elimination in these strains. Clodronate depletion of phagocytic cells compromises resistance of BALB/c mice and slows expulsion in the SJL strain. Thus, Type 2 immunity involves IL-4Rα-dependent innate cells including but not limited to a phagocyte population, the latter likely involving the action of specific antibodies.
Figures





Similar articles
-
Eosinophils and Macrophages within the Th2-Induced Granuloma: Balancing Killing and Healing in a Tight Space.Infect Immun. 2019 Sep 19;87(10):e00127-19. doi: 10.1128/IAI.00127-19. Print 2019 Oct. Infect Immun. 2019. PMID: 31285249 Free PMC article. Review.
-
Myeloid A20 is critical for alternative macrophage polarization and type-2 immune-mediated helminth resistance.Front Immunol. 2024 Apr 12;15:1373745. doi: 10.3389/fimmu.2024.1373745. eCollection 2024. Front Immunol. 2024. PMID: 38680500 Free PMC article.
-
Migratory CD103+ dendritic cells suppress helminth-driven type 2 immunity through constitutive expression of IL-12.J Exp Med. 2016 Jan 11;213(1):35-51. doi: 10.1084/jem.20150235. Epub 2015 Dec 28. J Exp Med. 2016. PMID: 26712805 Free PMC article.
-
Antibody-mediated trapping of helminth larvae requires CD11b and Fcγ receptor I.J Immunol. 2015 Feb 1;194(3):1154-63. doi: 10.4049/jimmunol.1401645. Epub 2014 Dec 29. J Immunol. 2015. PMID: 25548226 Free PMC article.
-
Context-dependent roles of B cells during intestinal helminth infection.PLoS Negl Trop Dis. 2021 May 13;15(5):e0009340. doi: 10.1371/journal.pntd.0009340. eCollection 2021 May. PLoS Negl Trop Dis. 2021. PMID: 33983946 Free PMC article. Review.
Cited by
-
Food for thought - ILC metabolism in the context of helminth infections.Mucosal Immunol. 2022 Jun;15(6):1234-1242. doi: 10.1038/s41385-022-00559-y. Epub 2022 Aug 31. Mucosal Immunol. 2022. PMID: 36045216 Free PMC article. Review.
-
Primary Heligmosomoides polygyrus bakeri infection induces myeloid-derived suppressor cells that suppress CD4+ Th2 responses and promote chronic infection.Mucosal Immunol. 2017 Jan;10(1):238-249. doi: 10.1038/mi.2016.36. Epub 2016 Apr 13. Mucosal Immunol. 2017. PMID: 27072608
-
Eosinophils and Macrophages within the Th2-Induced Granuloma: Balancing Killing and Healing in a Tight Space.Infect Immun. 2019 Sep 19;87(10):e00127-19. doi: 10.1128/IAI.00127-19. Print 2019 Oct. Infect Immun. 2019. PMID: 31285249 Free PMC article. Review.
-
A sticky end for gastrointestinal helminths; the role of the mucus barrier.Parasite Immunol. 2018 Apr;40(4):e12517. doi: 10.1111/pim.12517. Epub 2018 Mar 4. Parasite Immunol. 2018. PMID: 29355990 Free PMC article. Review.
-
Low-level regulatory T-cell activity is essential for functional type-2 effector immunity to expel gastrointestinal helminths.Mucosal Immunol. 2016 Mar;9(2):428-43. doi: 10.1038/mi.2015.73. Epub 2015 Aug 19. Mucosal Immunol. 2016. PMID: 26286232 Free PMC article.
References
-
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. - PubMed
-
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. - PubMed
-
- Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. - PubMed
-
- Turner J, Faulkner H, Kamgno J, Cormont F, Van Snick J, Else K, et al. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J Infect Dis. 2003;188:1768–1775. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

