GABAergic and glycinergic inputs modulate rhythmogenic mechanisms in the lamprey respiratory network
- PMID: 24492840
- PMCID: PMC4001755
- DOI: 10.1113/jphysiol.2013.268086
GABAergic and glycinergic inputs modulate rhythmogenic mechanisms in the lamprey respiratory network
Abstract
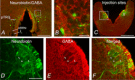
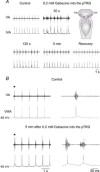
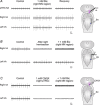
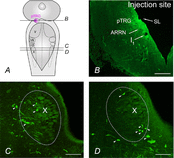
We have previously shown that GABA and glycine modulate respiratory activity in the in vitro brainstem preparations of the lamprey and that blockade of GABAA and glycine receptors restores the respiratory rhythm during apnoea caused by blockade of ionotropic glutamate receptors. However, the neural substrates involved in these effects are unknown. To address this issue, the role of GABAA, GABAB and glycine receptors within the paratrigeminal respiratory group (pTRG), the proposed respiratory central pattern generator, and the vagal motoneuron region was investigated both during apnoea induced by blockade of glutamatergic transmission and under basal conditions through microinjections of specific antagonists. The removal of GABAergic, but not glycinergic transmission within the pTRG, causes the resumption of rhythmic respiratory activity during apnoea, and reveals the presence of a modulatory control of the pTRG under basal conditions. A blockade of GABAA and glycine receptors within the vagal region strongly increases the respiratory frequency through disinhibition of neurons projecting to the pTRG from the vagal region. These neurons were retrogradely labelled (neurobiotin) from the pTRG. Intense GABA immunoreactivity is observed both within the pTRG and the vagal area, which corroborates present findings. The results confirm the pTRG as a primary site of respiratory rhythm generation, and suggest that inhibition modulates the activity of rhythm-generating neurons, without any direct role in burst formation and termination mechanisms.
Figures





Comment in
-
Lamprey breathing when feeding sucks: the respiratory rhythm generator of a parasitic fish.J Physiol. 2014 Apr 15;592(8):1725-6. doi: 10.1113/jphysiol.2014.272732. J Physiol. 2014. PMID: 24737896 Free PMC article. No abstract available.
References
-
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS) J Neurophysiol. 2008;99:1712–1722. - PubMed
-
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABAB receptors. Physiol Rev. 2004;84:835–867. - PubMed
-
- Bongianni F, Deliagina TG, Grillner S. Role of glutamate receptor subtypes in the lamprey respiratory network. Brain Res. 1999;826:298–302. - PubMed
-
- Bongianni F, Mutolo D, Carfi M, Pantaleo T. Group I and II metabotropic glutamate receptors modulate respiratory activity in the lamprey. Eur J Neurosci. 2002;16:454–460. - PubMed
-
- Bongianni F, Mutolo D, Cinelli E, Pantaleo T. Respiratory responses induced by blockades of GABA and glycine receptors within the Botzinger complex and the pre-Botzinger complex of the rabbit. Brain Res. 2010;1344:134–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

