Drosophila Tempura, a novel protein prenyltransferase α subunit, regulates notch signaling via Rab1 and Rab11
- PMID: 24492843
- PMCID: PMC3904817
- DOI: 10.1371/journal.pbio.1001777
Drosophila Tempura, a novel protein prenyltransferase α subunit, regulates notch signaling via Rab1 and Rab11
Abstract
Vesicular trafficking plays a key role in tuning the activity of Notch signaling. Here, we describe a novel and conserved Rab geranylgeranyltransferase (RabGGT)-α-like subunit that is required for Notch signaling-mediated lateral inhibition and cell fate determination of external sensory organs. This protein is encoded by tempura, and its loss affects the secretion of Scabrous and Delta, two proteins required for proper Notch signaling. We show that Tempura forms a heretofore uncharacterized RabGGT complex that geranylgeranylates Rab1 and Rab11. This geranylgeranylation is required for their proper subcellular localization. A partial dysfunction of Rab1 affects Scabrous and Delta in the secretory pathway. In addition, a partial loss Rab11 affects trafficking of Delta. In summary, Tempura functions as a new geranylgeranyltransferase that regulates the subcellular localization of Rab1 and Rab11, which in turn regulate trafficking of Scabrous and Delta, thereby affecting Notch signaling.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
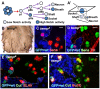

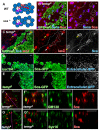




Comment in
-
Deep-fried signaling: tempura gets notch cooking.PLoS Biol. 2014 Jan 28;12(1):e1001778. doi: 10.1371/journal.pbio.1001778. eCollection 2014 Jan. PLoS Biol. 2014. PMID: 24492884 Free PMC article. No abstract available.
Similar articles
-
Deep-fried signaling: tempura gets notch cooking.PLoS Biol. 2014 Jan 28;12(1):e1001778. doi: 10.1371/journal.pbio.1001778. eCollection 2014 Jan. PLoS Biol. 2014. PMID: 24492884 Free PMC article. No abstract available.
-
Drosophila EHBP1 regulates Scabrous secretion during Notch-mediated lateral inhibition.J Cell Sci. 2013 Aug 15;126(Pt 16):3686-96. doi: 10.1242/jcs.126292. Epub 2013 Jun 20. J Cell Sci. 2013. PMID: 23788431 Free PMC article.
-
Rab11 plays a key role in stellate cell differentiation via non-canonical Notch pathway in Malpighian tubules of Drosophila melanogaster.Dev Biol. 2020 May 1;461(1):19-30. doi: 10.1016/j.ydbio.2020.01.002. Epub 2020 Jan 3. Dev Biol. 2020. PMID: 31911183
-
Robust selection of sensory organ precursors by the Notch-Delta pathway.Curr Opin Cell Biol. 2011 Dec;23(6):663-7. doi: 10.1016/j.ceb.2011.09.005. Epub 2011 Sep 29. Curr Opin Cell Biol. 2011. PMID: 21963301 Review.
-
Regulation of Notch Signaling in Drosophila melanogaster: The Role of the Heterogeneous Nuclear Ribonucleoprotein Hrp48 and Deltex.Adv Exp Med Biol. 2020;1227:95-105. doi: 10.1007/978-3-030-36422-9_7. Adv Exp Med Biol. 2020. PMID: 32072501 Review.
Cited by
-
Fruit flies in biomedical research.Genetics. 2015 Mar;199(3):639-53. doi: 10.1534/genetics.114.171785. Epub 2015 Jan 26. Genetics. 2015. PMID: 25624315 Free PMC article.
-
[Effects of RAB1A on the proliferation, invasion, and metastasis of tongue squamous cell carcinoma cells].Hua Xi Kou Qiang Yi Xue Za Zhi. 2020 Jun 1;38(3):245-249. doi: 10.7518/hxkq.2020.03.003. Hua Xi Kou Qiang Yi Xue Za Zhi. 2020. PMID: 32573129 Free PMC article. Chinese.
-
Regulation of NOTCH signaling by RAB7 and RAB8 requires carboxyl methylation by ICMT.J Cell Biol. 2017 Dec 4;216(12):4165-4182. doi: 10.1083/jcb.201701053. Epub 2017 Oct 19. J Cell Biol. 2017. PMID: 29051265 Free PMC article.
-
Amino acids-Rab1A-mTORC1 signaling controls whole-body glucose homeostasis.Cell Rep. 2021 Mar 16;34(11):108830. doi: 10.1016/j.celrep.2021.108830. Cell Rep. 2021. PMID: 33730578 Free PMC article.
-
Rab1 in cell signaling, cancer and other diseases.Oncogene. 2016 Nov 3;35(44):5699-5704. doi: 10.1038/onc.2016.81. Epub 2016 Apr 4. Oncogene. 2016. PMID: 27041585 Free PMC article. Review.
References
-
- Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689. - PubMed
-
- Artavanis-Tsakonas S, Muskavitch MA (2010) Notch: the past, the present, and the future. Curr Top Dev Biol 92: 1–29. - PubMed
-
- Gridley T (2003) Notch signaling and inherited disease syndromes. Hum Mol Genet 12 Spec No 1: R9–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

