Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy
- PMID: 24492943
- PMCID: PMC4083010
- DOI: 10.1002/hep.26996
Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy
Abstract
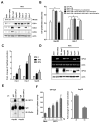
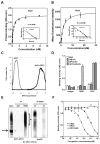
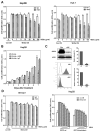
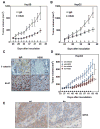
Wnt signaling is important for cancer pathogenesis and is often up-regulated in hepatocellular carcinoma (HCC). Heparan sulfate proteoglycans (HSPGs) function as coreceptors or modulators of Wnt activation. Glypican-3 (GPC3) is an HSPG that is highly expressed in HCC, where it can attract Wnt proteins to the cell surface and promote cell proliferation. Thus, GPC3 has emerged as a candidate therapeutic target in liver cancer. While monoclonal antibodies to GPC3 are currently being evaluated in preclinical and clinical studies, none have shown an effect on Wnt signaling. Here, we first document the expression of Wnt3a, multiple Wnt receptors, and GPC3 in several HCC cell lines, and demonstrate that GPC3 enhanced the activity of Wnt3a/β-catenin signaling in these cells. Then we report the identification of HS20, a human monoclonal antibody against GPC3, which preferentially recognized the heparan sulfate chains of GPC3, both the sulfated and nonsulfated portions. HS20 disrupted the interaction of Wnt3a and GPC3 and blocked Wnt3a/β-catenin signaling. Moreover, HS20 inhibited Wnt3a-dependent cell proliferation in vitro and HCC xenograft growth in nude mice. In addition, HS20 had no detectable undesired toxicity in mice. Taken together, our results show that a monoclonal antibody primarily targeting the heparin sulfate chains of GPC3 inhibited Wnt/β-catenin signaling in HCC cells and had potent antitumor activity in vivo.
Conclusion: An antibody directed against the heparan sulfate of a proteoglycan shows efficacy in blocking Wnt signaling and HCC growth, suggesting a novel strategy for liver cancer therapy.
Published 2014. This article is a U.S. Government work and is in the public domain in the USA.
Figures


Comment in
-
WNT/β-catenin signaling and hepatocellular carcinoma.Hepatology. 2014 Aug;60(2):452-4. doi: 10.1002/hep.27081. Epub 2014 Jun 18. Hepatology. 2014. PMID: 24644061 No abstract available.
References
-
- You XJ, Bryant PJ, Jurnak F, Holcombe RF. Expression of Wnt pathway components frizzled and disheveled in colon cancer arising in patients with inflammatory bowel disease. Oncol Rep. 2007;18:691–694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
