Pharmacokinetics of indomethacin in pregnancy
- PMID: 24493205
- PMCID: PMC4164383
- DOI: 10.1007/s40262-014-0133-6
Pharmacokinetics of indomethacin in pregnancy
Abstract
Background and objectives: Although indomethacin has been widely used for the treatment of preterm labor over the past 40 years, there are few reports regarding its pharmacokinetics in pregnant women.
Methods: This opportunistic study assessed the steady-state pharmacokinetics of indomethacin in pregnant subjects to whom an oral dose of 25 mg every 6 h was prescribed. Indomethacin concentrations in plasma and urine were analyzed by a validated high-performance liquid chromatography method with mass spectrometric detection.
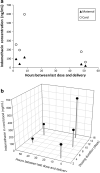
Results: The mean area under the plasma concentration versus time curve at steady state (AUCss) was 1.91 ± 0.53 μg·h/mL, mean peak plasma concentration (C max) was 1.02 ± 0.49 μg/mL, and mean time to reach C max (t max) was 1.3 ± 0.7 h. The mean apparent clearance at steady state was 14.5 ± 5.5 L/h, which is higher than the apparent clearance reported in the literature for non-pregnant subjects. Indomethacin crosses the placenta; the mean fetal/maternal ratio from five sets of cord blood samples collected at delivery was 4.0 ± 1.1.
Conclusions: Further studies are needed to determine whether any dose adjustments are necessary as a result of the increased clearance of indomethacin during pregnancy.
Figures

References
-
- Gamissans O, Balasch J. Prostaglandin synthetase inhibitors in the treatment of preterm birth. In: Fuchs A-R, Stubblefield PG, Fuchs F, editors. Preterm birth: causes, prevention, and management. 2nd ed. McGraw-Hill; New York: 1993. pp. 309–32.
-
- Zuckerman H, Shalev E, Gilad G, et al. Further study of the inhibition of premature labor by indomethacin. Part II: double-blind study. J Perinatal Med. 1984;12:25–9. - PubMed
-
- Zuckerman H, Reiss U, Rubinstein I. Inhibition of human premature labor by indomethacin. Obst Gynecol. 1974;44:787–92. - PubMed
-
- Wiqvist N. Endogenous prostaglandins in normal pregnancy and labor. Pharmacol Ther B. 1975;1:297–310. - PubMed
-
- Rane A, Oelz O, Frolich JC, et al. Relation between plasma concentration of indomethacin and its effect on prostaglandin synthesis and platelet aggregation in man. Clin Pharmacol Ther. 1978;23:658–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

