Role of anoctamins in cancer and apoptosis
- PMID: 24493744
- PMCID: PMC3917350
- DOI: 10.1098/rstb.2013.0096
Role of anoctamins in cancer and apoptosis
Abstract
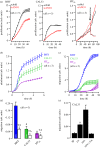
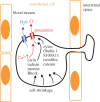
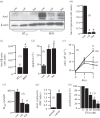
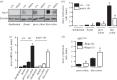
Anoctamin 1 (TMEM16A, Ano1) is a recently identified Ca(2+)-activated chloride channel and a member of a large protein family comprising 10 paralogues. Before Ano1 was identified as a chloride channel protein, it was known as the cancer marker DOG1. DOG1/Ano1 is expressed in gastrointestinal stromal tumours (GIST) and particularly in head and neck squamous cell carcinoma, at very high levels never detected in other tissues. It is now emerging that Ano1 is part of the 11q13 locus, amplified in several types of tumour, where it is thought to augment cell proliferation, cell migration and metastasis. Notably, Ano1 is upregulated through histone deacetylase (HDAC), corresponding to the known role of HDAC in HNSCC. As Ano1 does not enhance proliferation in every cell type, its function is perhaps modulated by cell-specific factors, or by the abundance of other anoctamins. Thus Ano6, by regulating Ca(2+)-induced membrane phospholipid scrambling and annexin V binding, supports cellular apoptosis rather than proliferation. Current findings implicate other cellular functions of anoctamins, apart from their role as Ca(2+)-activated Cl(-) channels.
Keywords: TMEM16A; TMEM16F; anoctamin 1; anoctamin 6; cancer; head and neck stromal cell carcinoma.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
