Regulation of voltage-gated sodium channel expression in cancer: hormones, growth factors and auto-regulation
- PMID: 24493753
- PMCID: PMC3917359
- DOI: 10.1098/rstb.2013.0105
Regulation of voltage-gated sodium channel expression in cancer: hormones, growth factors and auto-regulation
Abstract
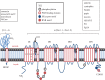
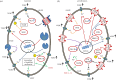
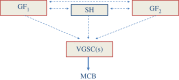
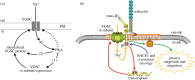
Although ion channels are increasingly being discovered in cancer cells in vitro and in vivo, and shown to contribute to different aspects and stages of the cancer process, much less is known about the mechanisms controlling their expression. Here, we focus on voltage-gated Na(+) channels (VGSCs) which are upregulated in many types of carcinomas where their activity potentiates cell behaviours integral to the metastatic cascade. Regulation of VGSCs occurs at a hierarchy of levels from transcription to post-translation. Importantly, mainstream cancer mechanisms, especially hormones and growth factors, play a significant role in the regulation. On the whole, in major hormone-sensitive cancers, such as breast and prostate cancer, there is a negative association between genomic steroid hormone sensitivity and functional VGSC expression. Activity-dependent regulation by positive feedback has been demonstrated in strongly metastatic cells whereby the VGSC is self-sustaining, with its activity promoting further functional channel expression. Such auto-regulation is unlike normal cells in which activity-dependent regulation occurs mostly via negative feedback. Throughout, we highlight the possible clinical implications of functional VGSC expression and regulation in cancer.
Keywords: activity-dependent regulation; growth factor; hormone; metastasis; voltage-gated sodium channel.
Figures


References
-
- Djamgoz MBA. 2011. Bioelectricity of cancer: voltage-gated ion channels and direct-current electric fields. In The physiology of bioelectricity in development, tissue regeneration, and cancer (ed. Pullar C.), pp. 269–294. London, UK: Taylor & Francis.
-
- Djamgoz MBA, Onkal R. 2013. Persistent current blockers of voltage-gated sodium channels: a clinical opportunity for controlling metastatic disease. Recent Patents Anti-Cancer Drug Discov. 8, 66–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
