Ion channels and transporters in the development of drug resistance in cancer cells
- PMID: 24493757
- PMCID: PMC3917363
- DOI: 10.1098/rstb.2013.0109
Ion channels and transporters in the development of drug resistance in cancer cells
Abstract
Multi-drug resistance (MDR) to chemotherapy is the major challenge in the treatment of cancer. MDR can develop by numerous mechanisms including decreased drug uptake, increased drug efflux and the failure to undergo drug-induced apoptosis. Evasion of drug-induced apoptosis through modulation of ion transporters is the main focus of this paper and we demonstrate how pro-apoptotic ion channels are downregulated, while anti-apoptotic ion transporters are upregulated in MDR. We also discuss whether upregulation of ion transport proteins that are important for proliferation contribute to MDR. Finally, we discuss the possibility that the development of MDR involves sequential and localized upregulation of ion channels involved in proliferation and migration and a concomitant global and persistent downregulation of ion channels involved in apoptosis.
Keywords: apoptosis; cancer; drug resistance; ion channels in cancer; tumour proliferation.
Figures


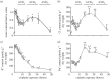
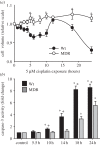
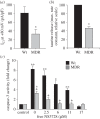
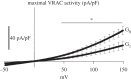
References
-
- Stavrovskaya AA. 2000. Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry (Mosc). 65, 95–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
