Dynamics and activation in response regulators: the β4-α4 loop
- PMID: 24494032
- PMCID: PMC3909562
- DOI: 10.1515/bmc-2011-0063
Dynamics and activation in response regulators: the β4-α4 loop
Abstract
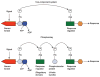
Two-component signal transduction systems of microbes are a primary means to respond to signals emanating from environmental and metabolic fluctuations as well as to signals coordinating the cell cycle with macromolecular syntheses, among a large variety of other essential roles. Signals are recognized by a sensor domain of a histidine kinase which serves to convert signal binding to an active transmissible phosphoryl group through a signal-induced ATP-dependent autophosphorylation reaction directed to histidine residue. The sensor kinase is specifically mated to a response regulator, to which it transfers the phosphoryl group that activates the response regulator's function, most commonly gene repression or activation but also interaction with other regulatory proteins. Two-component systems have been genetically amplified to control a wide variety of cellular processes; for example, both Escherichia coli and Pseudomonas aeruginosa have 60 plus confirmed and putative two-component systems. Bacillus subtilis has 30 plus and Nostoc punctiformis over 100. As genetic amplification does not result in changes in the basic structural folds of the catalytic domains of the sensor kinase or response regulators, each sensor kinase must recognize its partner through subtle changes in residues at the interaction surface between the two proteins. Additionally, the response regulator must prepare itself for efficient activation by the phosphorylation event. In this short review, we discuss the contributions of the critical β4-α4 recognition loop in response regulators to their function. In particular, we focus on this region's microsecond-millisecond timescale dynamics propensities and discuss how these motions play a major role in response regulator recognition and activation.
Keywords: NMR; millisecond dynamics; phosphorylation; response regulator; β4-α4 recognition loop.
Figures


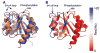




Similar articles
-
A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction.Structure. 2000 Aug 15;8(8):851-62. doi: 10.1016/s0969-2126(00)00174-x. Structure. 2000. PMID: 10997904
-
The Histidine Residue of QseC Is Required for Canonical Signaling between QseB and PmrB in Uropathogenic Escherichia coli.J Bacteriol. 2017 Aug 22;199(18):e00060-17. doi: 10.1128/JB.00060-17. Print 2017 Sep 15. J Bacteriol. 2017. PMID: 28396353 Free PMC article.
-
Allosteric activation of bacterial response regulators: the role of the cognate histidine kinase beyond phosphorylation.mBio. 2014 Nov 18;5(6):e02105. doi: 10.1128/mBio.02105-14. mBio. 2014. PMID: 25406381 Free PMC article.
-
Aspartyl-phosphate phosphatases deactivate the response regulator components of the sporulation signal transduction system in Bacillus subtilis.Mol Microbiol. 1996 Mar;19(6):1151-7. doi: 10.1111/j.1365-2958.1996.tb02460.x. Mol Microbiol. 1996. PMID: 8730857 Review.
-
Signal Sensing and Transduction by Histidine Kinases as Unveiled through Studies on a Temperature Sensor.Acc Chem Res. 2017 Jun 20;50(6):1359-1366. doi: 10.1021/acs.accounts.6b00593. Epub 2017 May 5. Acc Chem Res. 2017. PMID: 28475313 Review.
Cited by
-
Structure of the Francisella response regulator QseB receiver domain, and characterization of QseB inhibition by antibiofilm 2-aminoimidazole-based compounds.Mol Microbiol. 2017 Oct;106(2):223-235. doi: 10.1111/mmi.13759. Epub 2017 Aug 16. Mol Microbiol. 2017. PMID: 28755524 Free PMC article.
-
Conformational Dynamics of Response Regulator RegX3 from Mycobacterium tuberculosis.PLoS One. 2015 Jul 22;10(7):e0133389. doi: 10.1371/journal.pone.0133389. eCollection 2015. PLoS One. 2015. PMID: 26201027 Free PMC article.
-
Use of restrained molecular dynamics to predict the conformations of phosphorylated receiver domains in two-component signaling systems.Proteins. 2017 Jan;85(1):155-176. doi: 10.1002/prot.25207. Epub 2016 Nov 20. Proteins. 2017. PMID: 27802580 Free PMC article.
-
The Structure of the Biofilm-controlling Response Regulator BfmR from Acinetobacter baumannii Reveals Details of Its DNA-binding Mechanism.J Mol Biol. 2018 Mar 16;430(6):806-821. doi: 10.1016/j.jmb.2018.02.002. Epub 2018 Feb 10. J Mol Biol. 2018. PMID: 29438671 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
