Novel vitamin D photoproducts and their precursors in the skin
- PMID: 24494038
- PMCID: PMC3897599
- DOI: 10.4161/derm.23938
Novel vitamin D photoproducts and their precursors in the skin
Abstract
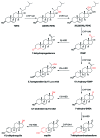
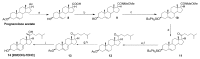
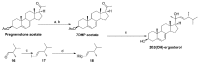
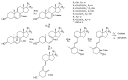
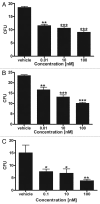
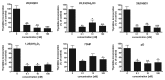
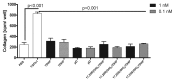
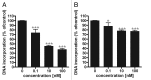
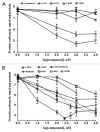
Novel metabolic pathways initiated by the enzymatic action of CYP11A1 on 7DHC (7-dehydrocholesterol), ergosterol, vitamins D3 and D2 were characterized with help of chemical synthesis, UV and mass spectrometry and NMR analyses. The first pathway follows the sequence 7DHC→22(OH)7DHC → 20,22(OH)27DHC → 7DHP (7-dehydropregnenolone), which can further be metabolized by steroidogenic enzymes. The resulting 5,7-dienes can be transformed by UVB to corresponding, biologically active, secosteroids. Action of CYP11A1 on vitamin D3 and D2 produces novel hydroxyderivatives with OH added at positions C17, C20, C22, C23 and C24, some of which can be hydroxylated by CYP27B1 and/or by CYP27A1 and/ or by CYP24A1.The main products of these pathways are biologically active with a potency related to their chemical structure and the target cell type. Main products of CYP11A1-mediated metabolism on vitamin D are non-calcemic and non-toxic at relatively high doses and serve as partial agonists on the vitamin D receptor. New secosteroids are excellent candidates for therapy of fibrosing, inflammatory or hyperproliferative disorders including cancers and psoriasis.
Keywords: 5,7-dienes; dermal fibroblasts; keratinocytes; melanocytes; melanoma cells; skin; vitamin D.
Figures
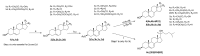
Similar articles
-
Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives.Cell Biochem Biophys. 2020 Jun;78(2):165-180. doi: 10.1007/s12013-020-00913-6. Epub 2020 May 22. Cell Biochem Biophys. 2020. PMID: 32441029 Free PMC article. Review.
-
The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions.J Steroid Biochem Mol Biol. 2014 Oct;144 Pt A:28-39. doi: 10.1016/j.jsbmb.2013.10.012. Epub 2013 Oct 28. J Steroid Biochem Mol Biol. 2014. PMID: 24176765 Free PMC article. Review.
-
Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin.PLoS One. 2009;4(2):e4309. doi: 10.1371/journal.pone.0004309. Epub 2009 Feb 3. PLoS One. 2009. PMID: 19190754 Free PMC article.
-
Cytochromes p450 and skin cancer: role of local endocrine pathways.Anticancer Agents Med Chem. 2014 Jan;14(1):77-96. doi: 10.2174/18715206113139990308. Anticancer Agents Med Chem. 2014. PMID: 23869782 Free PMC article. Review.
-
Selective ability of rat 7-Dehydrocholesterol reductase (DHCR7) to act on some 7-Dehydrocholesterol metabolites but not on lumisterol metabolites.J Steroid Biochem Mol Biol. 2021 Sep;212:105929. doi: 10.1016/j.jsbmb.2021.105929. Epub 2021 Jun 11. J Steroid Biochem Mol Biol. 2021. PMID: 34098080 Free PMC article.
Cited by
-
Novel CYP11A1-Derived Vitamin D and Lumisterol Biometabolites for the Management of COVID-19.Nutrients. 2022 Nov 11;14(22):4779. doi: 10.3390/nu14224779. Nutrients. 2022. PMID: 36432468 Free PMC article. Review.
-
Editorial: Steroids and Secosteroids in the Modulation of Inflammation and Immunity.Front Immunol. 2021 Dec 20;12:825577. doi: 10.3389/fimmu.2021.825577. eCollection 2021. Front Immunol. 2021. PMID: 34987528 Free PMC article. No abstract available.
-
Design, Synthesis and Biological Activities of Novel Gemini 20S-Hydroxyvitamin D3 Analogs.Anticancer Res. 2016 Mar;36(3):877-86. Anticancer Res. 2016. PMID: 26976974 Free PMC article.
-
Illuminating the Connection: Cutaneous Vitamin D3 Synthesis and Its Role in Skin Cancer Prevention.Nutrients. 2025 Jan 22;17(3):386. doi: 10.3390/nu17030386. Nutrients. 2025. PMID: 39940244 Free PMC article. Review.
-
Vitamin D and hypertension: Prospective study and meta-analysis.PLoS One. 2017 Mar 30;12(3):e0174298. doi: 10.1371/journal.pone.0174298. eCollection 2017. PLoS One. 2017. PMID: 28358827 Free PMC article.
References
-
- Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
