Effects of sidechain length and composition on the kinetic conversion and product distribution of vitamin D analogs determined by real-time NMR
- PMID: 24494047
- PMCID: PMC3897582
- DOI: 10.4161/derm.24339
Effects of sidechain length and composition on the kinetic conversion and product distribution of vitamin D analogs determined by real-time NMR
Abstract
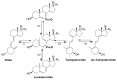
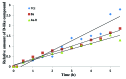
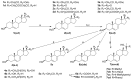
Novel pregna-5, 7-dienes were synthesized and subjected to UVB irradiation to generate the corresponding pre-D intermediates, tachysterol and lumisterol analogs. The kinetics of the conversion from each of the pre-D intermediates to the corresponding novel D analogs was investigated by using real time 1H NMR measurements inside the NMR magnet. Both the length and composition of the side chains were found to affect the rate of the kinetic conversion from pre-D intermediates to the thermodynamically more stable D analogs. Compound 7cc which has both a long side chain and a tertiary alcohol moiety showed the highest conversion rate, while compound 4a-S which has a very short side chain without the tertiary alcohol had the lowest conversion rate among the 13 tested compounds. We also determined product distributions for these 5,7-dienes upon UVB irradiation followed by thermodynamic equilibration. No clear correlations between product distribution and side chain length or composition were identifiable under the current experimental conditions, suggesting there are other factors affecting the kinetics during the photochemical reactions for these 5,7-dienes. To the best of our knowledge, this is the first time the influences of side chain length and composition on the real time conversion kinetics from pre-D to D are studied. This study could serve as step-stones in future kinetic studies of novel biologically active 5,7-dienes and their corresponding D analogs under more physiologically relevant ex vivo or in vivo conditions, as well as providing important insights into optimizing yields of the desired active products during their organic syntheses.
Keywords: UVB; kinetics; pre-D; real-time NMR; vitamin D.
Figures

Similar articles
-
Synthesis and photo-conversion of androsta- and pregna-5,7-dienes to vitamin D3-like derivatives.Photochem Photobiol Sci. 2008 Dec;7(12):1570-6. doi: 10.1039/b809005j. Epub 2008 Sep 4. Photochem Photobiol Sci. 2008. PMID: 19037511 Free PMC article.
-
Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells.Steroids. 2009 Feb;74(2):218-28. doi: 10.1016/j.steroids.2008.10.017. Epub 2008 Nov 6. Steroids. 2009. PMID: 19028513 Free PMC article.
-
Novel side chain analogs of 1alpha,25-dihydroxyvitamin D(3): design and synthesis of the 21,24-methano derivatives.Steroids. 2001 Mar-May;66(3-5):249-55. doi: 10.1016/s0039-128x(00)00156-2. Steroids. 2001. PMID: 11179732
-
Design, synthesis, and biological studies of the A-ring-modified 1,25-dihydroxyvitamin D3 analogs.Recent Results Cancer Res. 2003;164:289-317. doi: 10.1007/978-3-642-55580-0_21. Recent Results Cancer Res. 2003. PMID: 12899530 Review.
-
Novel vitamin D photoproducts and their precursors in the skin.Dermatoendocrinol. 2013 Jan 1;5(1):7-19. doi: 10.4161/derm.23938. Dermatoendocrinol. 2013. PMID: 24494038 Free PMC article. Review.
Cited by
-
Novel vitamin D analogs as potential therapeutics: metabolism, toxicity profiling, and antiproliferative activity.Anticancer Res. 2014 May;34(5):2153-63. Anticancer Res. 2014. PMID: 24778017 Free PMC article.
-
The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions.J Steroid Biochem Mol Biol. 2014 Oct;144 Pt A:28-39. doi: 10.1016/j.jsbmb.2013.10.012. Epub 2013 Oct 28. J Steroid Biochem Mol Biol. 2014. PMID: 24176765 Free PMC article. Review.
-
Biological Effects of CYP11A1-Derived Vitamin D and Lumisterol Metabolites in the Skin.J Invest Dermatol. 2024 Oct;144(10):2145-2161. doi: 10.1016/j.jid.2024.04.022. Epub 2024 Jul 12. J Invest Dermatol. 2024. PMID: 39001720 Review.
-
On the role of classical and novel forms of vitamin D in melanoma progression and management.J Steroid Biochem Mol Biol. 2018 Mar;177:159-170. doi: 10.1016/j.jsbmb.2017.06.013. Epub 2017 Jul 1. J Steroid Biochem Mol Biol. 2018. PMID: 28676457 Free PMC article. Review.
-
Safety Assessment of Vitamin D and Its Photo-Isomers in UV-Irradiated Baker's Yeast.Foods. 2021 Dec 18;10(12):3142. doi: 10.3390/foods10123142. Foods. 2021. PMID: 34945693 Free PMC article. Review.
References
-
- Holick MF, Clark MB. The photobiogenesis and metabolism of vitamin D. Fed Proc. 1978;37:2567–74. - PubMed
-
- Slominski A, Li W, Zbytek B, Tuckey RC, Zjawiony J, Nguyen MN, et al. Enzymatic production or chemical synthesis and uses for 5,7-dienes and UVB conversion products thereof. US2011/0118228A1, 2011.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
