Mitochondria-targeted heme oxygenase-1 induces oxidative stress and mitochondrial dysfunction in macrophages, kidney fibroblasts and in chronic alcohol hepatotoxicity
- PMID: 24494190
- PMCID: PMC3909819
- DOI: 10.1016/j.redox.2013.07.004
Mitochondria-targeted heme oxygenase-1 induces oxidative stress and mitochondrial dysfunction in macrophages, kidney fibroblasts and in chronic alcohol hepatotoxicity
Abstract
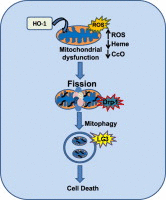
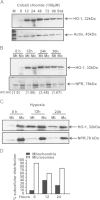
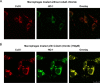
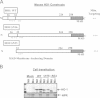
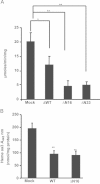
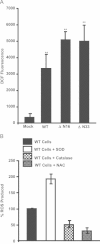
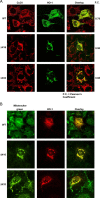
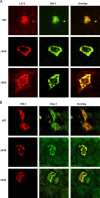
The inducible form of Heme Oxygenase-1 (HO-1), a major endoplasmic reticulum (ER) associated heme protein, is known to play important roles in protection against oxidative and chemical stress by degrading free heme released from degradation of heme proteins. In this study we show that induced expression of HO-1 by subjecting macrophage RAW-264.7 cells to chemical or physiological hypoxia resulted in significant translocation of HO-1 protein to mitochondria. Transient transfection of COS-7 cells with cloned cDNA also resulted in mitochondrial translocation of HO-1. Deletion of N-terminal ER targeting domain increased mitochondrial translocation under the transient transfection conditions. Mitochondrial localization of both intact HO-1 and N-terminal truncated HO-1 caused loss of heme aa-3 and cytochrome c oxidase (CcO) activity in COS-7 cells. The truncated protein, which localizes to mitochondria at higher levels, induced substantially steeper loss of CcO activity and reduced heme aa3 content. Furthermore, cells expressing mitochondria targeted HO-1 also induced higher ROS production. Consistent with dysfunctional state of mitochondria induced by HO-1, the mitochondrial recruitment of autophagy markers LC-3 and Drp-1 was also increased in these cells. Chronic ethanol feeding in rats also caused an increase in mitochondrial HO-1 and decrease in CcO activity. These results show that as opposed to the protective effect of the ER associated HO-1, mitochondria targeted HO-1 under normoxic conditions induces mitochondrial dysfunction.
Keywords: Autophagy; CcO, cytochrome c oxidase; Cytochrome c Oxidase; DCFH-DA, Dichlorofluorescein diacetate; ER, Endoplasmic reticulum; HO-1, Heme Oxygenase-1; Heme aa3 content; Heme oxygenase-1; Mitochondrial targeting; NPR, NADPH cytochrome P 450 reductase; ROS production; ROS, Reactive Oxygen Species.
Figures

References
-
- Snyder S.H., Baranano D.E. Heme oxygenase: a font of multiple messengers. Neuropsychopharmacology. 2001;25:294–298. - PubMed
-
- Abraham N.G., Lin J.H., Schwartzman M.L., Levere R.D., Shibahara S. The physiological significance of heme oxygenase. Int. J. Biochem. 1988;20:543–558. - PubMed
-
- Maines M.D. The heme oxygenase system: past, present, and future. Antioxid. Redox Signal. 2004;6:797–801. - PubMed
-
- Ryter S.W., Tyrrell R.M. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic. Biol. Med. 2000;28:289–309. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

