Deletion of the Kv2.1 delayed rectifier potassium channel leads to neuronal and behavioral hyperexcitability
- PMID: 24494598
- PMCID: PMC4077602
- DOI: 10.1111/gbb.12120
Deletion of the Kv2.1 delayed rectifier potassium channel leads to neuronal and behavioral hyperexcitability
Abstract
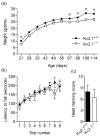
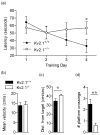
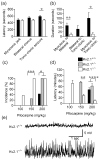
The Kv2.1 delayed rectifier potassium channel exhibits high-level expression in both principal and inhibitory neurons throughout the central nervous system, including prominent expression in hippocampal neurons. Studies of in vitro preparations suggest that Kv2.1 is a key yet conditional regulator of intrinsic neuronal excitability, mediated by changes in Kv2.1 expression, localization and function via activity-dependent regulation of Kv2.1 phosphorylation. Here we identify neurological and behavioral deficits in mutant (Kv2.1(-/-) ) mice lacking this channel. Kv2.1(-/-) mice have grossly normal characteristics. No impairment in vision or motor coordination was apparent, although Kv2.1(-/-) mice exhibit reduced body weight. The anatomic structure and expression of related Kv channels in the brains of Kv2.1(-/-) mice appear unchanged. Delayed rectifier potassium current is diminished in hippocampal neurons cultured from Kv2.1(-/-) animals. Field recordings from hippocampal slices of Kv2.1(-/-) mice reveal hyperexcitability in response to the convulsant bicuculline, and epileptiform activity in response to stimulation. In Kv2.1(-/-) mice, long-term potentiation at the Schaffer collateral - CA1 synapse is decreased. Kv2.1(-/-) mice are strikingly hyperactive, and exhibit defects in spatial learning, failing to improve performance in a Morris Water Maze task. Kv2.1(-/-) mice are hypersensitive to the effects of the convulsants flurothyl and pilocarpine, consistent with a role for Kv2.1 as a conditional suppressor of neuronal activity. Although not prone to spontaneous seizures, Kv2.1(-/-) mice exhibit accelerated seizure progression. Together, these findings suggest homeostatic suppression of elevated neuronal activity by Kv2.1 plays a central role in regulating neuronal network function.
Keywords: Hyperactivity; Kcnb1; Kcnb1tm1Dgen; long-term potentiation; seizure.
© 2014 John Wiley & Sons Ltd and International Behavioural and Neural Genetics Society.
Figures





References
-
- Antonucci DE, Lim ST, Vassanelli S, Trimmer JS. Dynamic localization and clustering of dendritic Kv2.1 voltage-dependent potassium channels in developing hippocampal neurons. Neuroscience. 2001;108:69–81. - PubMed
-
- Ariano MA, Cepeda C, Calvert CR, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Chandler SH, Aronin N, DiFiglia M, Levine MS. Striatal potassium channel dysfunction in Huntington’s disease transgenic mice. J Neurophysiol. 2005;93:2565–2574. - PubMed
-
- Beaudoin GM, 3rd, Lee SH, Singh D, Yuan Y, Ng YG, Reichardt LF, Arikkath J. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nature protocols. 2012;7:1741–1754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

