Suppression of neuroinflammation in forebrain-specific Cdk5 conditional knockout mice by PPARγ agonist improves neuronal loss and early lethality
- PMID: 24495352
- PMCID: PMC3931315
- DOI: 10.1186/1742-2094-11-28
Suppression of neuroinflammation in forebrain-specific Cdk5 conditional knockout mice by PPARγ agonist improves neuronal loss and early lethality
Abstract
Background: Cyclin-dependent kinase 5 (Cdk5) is essential for brain development and function, and its deregulated expression is implicated in some of neurodegenerative diseases. We reported earlier that the forebrain-specific Cdk5 conditional knockout (cKO) mice displayed an early lethality associated with neuroinflammation, increased expression of the neuronal tissue-type plasminogen activator (tPA), and neuronal migration defects.
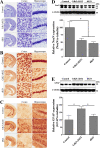
Methods: In order to suppress neuroinflammation in the cKO mice, we first treated these mice with pioglitazone, a PPARγ agonist, and analyzed its effects on neuronal loss and longevity. In a second approach, to delineate the precise role of tPA in neuroinflammation in these mice, we generated Cdk5 cKO; tPA double knockout (dKO) mice.
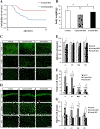
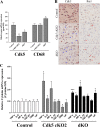
Results: We found that pioglitazone treatment significantly reduced astrogliosis, microgliosis, neuronal loss and behavioral deficit in Cdk5 cKO mice. Interestingly, the dKO mice displayed a partial reversal in astrogliosis, but they still died at early age, suggesting that the increased expression of tPA in the cKO mice does not contribute significantly to the pathological process leading to neuroinflammation, neuronal loss and early lethality.
Conclusion: The suppression of neuroinflammation in Cdk5 cKO mice ameliorates gliosis and neuronal loss, thus suggesting the potential beneficial effects of the PPARγ agonist pioglitazone for the treatment for neurodegenerative diseases.
Figures

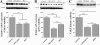
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

