Modulation of nuclear receptor function by cellular redox poise
- PMID: 24495544
- PMCID: PMC3986263
- DOI: 10.1016/j.jinorgbio.2014.01.005
Modulation of nuclear receptor function by cellular redox poise
Abstract
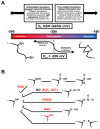
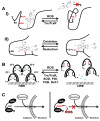
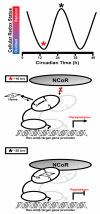
Nuclear receptors (NRs) are ligand-responsive transcription factors involved in diverse cellular processes ranging from metabolism to circadian rhythms. This review focuses on NRs that contain redox-active thiol groups, a common feature within the superfamily. We will begin by describing NRs, how they regulate various cellular processes and how binding ligands, corepressors and/or coactivators modulate their activity. We will then describe the general area of redox regulation, especially as it pertains to thiol-disulfide interconversion and the cellular systems that respond to and govern this redox equilibrium. Lastly, we will discuss specific examples of NRs whose activities are regulated by redox-active thiols. Glucocorticoid, estrogen, and the heme-responsive receptor, Rev-erb, will be described in the most detail as they exhibit archetypal redox regulatory mechanisms.
Keywords: Hormone; Nuclear receptor; Oxidative stress; Redox; Thiol–disulfide; Transcription.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
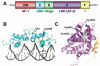
Similar articles
-
Thiol-disulfide redox dependence of heme binding and heme ligand switching in nuclear hormone receptor rev-erb{beta}.J Biol Chem. 2011 Feb 11;286(6):4392-403. doi: 10.1074/jbc.M110.193466. Epub 2010 Dec 1. J Biol Chem. 2011. PMID: 21123168 Free PMC article.
-
Thiol/Disulfide redox switches in the regulation of heme binding to proteins.Antioxid Redox Signal. 2011 Mar 15;14(6):1039-47. doi: 10.1089/ars.2010.3436. Epub 2010 Dec 27. Antioxid Redox Signal. 2011. PMID: 20812781 Free PMC article. Review.
-
Heme regulatory motifs in heme oxygenase-2 form a thiol/disulfide redox switch that responds to the cellular redox state.J Biol Chem. 2009 Jul 31;284(31):20556-61. doi: 10.1074/jbc.M109.015651. Epub 2009 May 27. J Biol Chem. 2009. PMID: 19473966 Free PMC article.
-
Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE.J Biol Chem. 2004 Nov 12;279(46):47939-51. doi: 10.1074/jbc.M408011200. Epub 2004 Aug 30. J Biol Chem. 2004. PMID: 15347644
-
Thiols in cellular redox signalling and control.Curr Med Chem. 2001 Jun;8(7):763-72. doi: 10.2174/0929867013372904. Curr Med Chem. 2001. PMID: 11375748 Review.
Cited by
-
Redox Regulation of Heme Oxygenase-2 and the Transcription Factor, Rev-Erb, Through Heme Regulatory Motifs.Antioxid Redox Signal. 2018 Dec 20;29(18):1841-1857. doi: 10.1089/ars.2017.7368. Epub 2017 Nov 14. Antioxid Redox Signal. 2018. PMID: 28990415 Free PMC article. Review.
-
Ferric heme as a CO/NO sensor in the nuclear receptor Rev-Erbß by coupling gas binding to electron transfer.Proc Natl Acad Sci U S A. 2021 Jan 19;118(3):e2016717118. doi: 10.1073/pnas.2016717118. Proc Natl Acad Sci U S A. 2021. PMID: 33436410 Free PMC article.
-
Regulation of protein function and degradation by heme, heme responsive motifs, and CO.Crit Rev Biochem Mol Biol. 2022 Feb;57(1):16-47. doi: 10.1080/10409238.2021.1961674. Epub 2021 Sep 13. Crit Rev Biochem Mol Biol. 2022. PMID: 34517731 Free PMC article. Review.
-
Cellular Timekeeping: It's Redox o'Clock.Cold Spring Harb Perspect Biol. 2018 May 1;10(5):a027698. doi: 10.1101/cshperspect.a027698. Cold Spring Harb Perspect Biol. 2018. PMID: 28778867 Free PMC article. Review.
-
High Affinity Heme Binding to a Heme Regulatory Motif on the Nuclear Receptor Rev-erbβ Leads to Its Degradation and Indirectly Regulates Its Interaction with Nuclear Receptor Corepressor.J Biol Chem. 2016 Jan 29;291(5):2196-222. doi: 10.1074/jbc.M115.670281. Epub 2015 Dec 15. J Biol Chem. 2016. PMID: 26670607 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

