Characterization of the biological anti-staphylococcal functionality of hUK-66 IgG1, a humanized monoclonal antibody as substantial component for an immunotherapeutic approach
- PMID: 24495867
- PMCID: PMC4896585
- DOI: 10.4161/hv.27692
Characterization of the biological anti-staphylococcal functionality of hUK-66 IgG1, a humanized monoclonal antibody as substantial component for an immunotherapeutic approach
Abstract
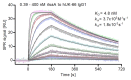
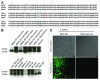
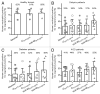
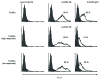
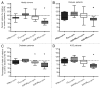
Multi-antigen immunotherapy approaches against Staphylococcus aureus are expected to have the best chance of clinical success when used in combinatorial therapy, potentially incorporating opsonic killing of bacteria and toxin neutralization. We recently reported the development of a murine monoclonal antibody specific for the immunodominant staphylococcal antigen A (IsaA), which showed highly efficient staphylococcal killing in experimental infection models of S. aureus. If IsaA-specific antibodies are to be used as a component of combination therapy in humans, the binding specificity and biological activity of the humanized variant must be preserved. Here, we describe the functional characterization of a humanized monoclonal IgG1 variant designated, hUK-66. The humanized antibody showed comparable binding kinetics to those of its murine parent, and recognized the target antigen IsaA on the surface of clinically relevant S. aureus lineages. Furthermore, hUK-66 enhances the killing of S. aureus in whole blood (a physiological environment) samples from healthy subjects and patients prone to staphylococcal infections such as diabetes and dialysis patients, and patients with generalized artery occlusive disease indicating no interference with already present natural antibodies. Taken together, these data indicate that hUK-66 mediates bacterial killing even in high risk patients and thus, could play a role for immunotherapy strategies to combat severe S. aureus infections.
Keywords: MRSA; Staphylococcus aureus; bacterial killing; fc-receptors; humanization; immunotherapeutics; immunotherapy; monoclonal antibodies; opsonophagocytosis; vaccinology.
Figures



References
-
- Sánchez García M. . Early antibiotic treatment failure. Int J Antimicrob Agents 2009; 34:Suppl 3 S14 - 9; http://dx.doi.org/10.1016/S0924-8579(09)70552-7; PMID: 19596109 - DOI - PubMed
-
- Rubinstein E, Kollef MH, Nathwani D. . Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2008; 46:Suppl 5 S378 - 85; http://dx.doi.org/10.1086/533594; PMID: 18462093 - DOI - PubMed
-
- Kollef MH, Morrow LE, Niederman MS, Leeper KV, Anzueto A, Benz-Scott L, Rodino FJ. . Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest 2006; 129:1210 - 8; http://dx.doi.org/10.1378/chest.129.5.1210; PMID: 16685011 - DOI - PubMed
-
- Verkaik NJ, van Wamel WJ, van Belkum A. . Immunotherapeutic approaches against Staphylococcus aureus.. Immunotherapy 2011; 3:1063 - 73; http://dx.doi.org/10.2217/imt.11.84; PMID: 21913829 - DOI - PubMed
-
- Jansen KU, Girgenti DQ, Scully IL, Anderson AS. . Vaccine review: “Staphyloccocus aureus vaccines: problems and prospects”. Vaccine 2013; 31:2723 - 30; http://dx.doi.org/10.1016/j.vaccine.2013.04.002; PMID: 23624095 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
