Differences in antibody responses between trivalent inactivated influenza vaccine and live attenuated influenza vaccine correlate with the kinetics and magnitude of interferon signaling in children
- PMID: 24495909
- PMCID: PMC4092249
- DOI: 10.1093/infdis/jiu079
Differences in antibody responses between trivalent inactivated influenza vaccine and live attenuated influenza vaccine correlate with the kinetics and magnitude of interferon signaling in children
Abstract
Background: Live attenuated influenza vaccine (LAIV) and trivalent inactivated influenza vaccine (TIV) are effective for prevention of influenza virus infection in children, but the mechanisms associated with protection are not well defined.
Methods: We analyzed the differences in B-cell responses and transcriptional profiles in children aged 6 months to 14 years immunized with these 2 vaccines.
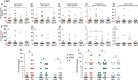
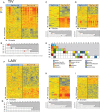
Results: LAIV elicited a significant increase in naive, memory, and transitional B cells on day 30 after vaccination, whereas TIV elicited an increased number of plasmablasts on day 7. Antibody titers against the 3 vaccine strains (H1N1, H3N2, and B) were significantly higher in the TIV group and correlated with number of antibody-secreting cells. Both vaccines induced overexpression of interferon (IFN)-signaling genes but with different kinetics. TIV induced expression of IFN genes on day 1 after vaccination in all age groups, and LAIV induced expression of IFN genes on day 7 after vaccination but only in children <5 years old. IFN-related genes overexpressed in both vaccinated groups correlated with H3N2 antibody titers.
Conclusions: These results suggest that LAIV and TIV induced significantly different B-cell responses in vaccinated children. Early induction of IFN appears to be important for development of antibody responses.
Keywords: HI antibodies; Influenza vaccine; LAIV; TIV; children; interferon; neutralizing antibodies.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342:232–9. - PubMed
-
- Neuzil KM, Zhu Y, Griffin MR, et al. Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. J Infect Dis. 2002;185:147–52. - PubMed
-
- Heikkinen T, Silvennoinen H, Peltola V, et al. Burden of influenza in children in the community. J Infect Dis. 2004;190:1369–73. - PubMed
-
- Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. - PubMed
-
- Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

