The extracellular matrix protein artichoke is required for integrity of ciliated mechanosensory and chemosensory organs in Drosophila embryos
- PMID: 24496014
- PMCID: PMC3982677
- DOI: 10.1534/genetics.113.156323
The extracellular matrix protein artichoke is required for integrity of ciliated mechanosensory and chemosensory organs in Drosophila embryos
Abstract
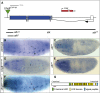
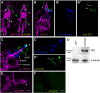
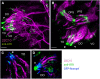
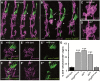
Sensory cilia are often encapsulated by an extracellular matrix (ECM). In Caenorhabditis elegans, Drosophila melanogaster, and vertebrates, this ECM is thought to be directly involved in ciliary mechanosensing by coupling external forces to the ciliary membrane. Drosophila mechano- and chemosensory cilia are both associated with an ECM, indicating that the ECM may have additional roles that go beyond mechanosensory cilium function. Here, we identify Artichoke (ATK), an evolutionarily conserved leucine-rich repeat ECM protein that is required for normal morphogenesis and function of ciliated sensilla in Drosophila. atk is transiently expressed in accessory cells in all ciliated sensory organs during their late embryonic development. Antibody stainings show ATK protein in the ECM that surrounds sensory cilia. Loss of ATK protein in atk null mutants leads to cilium deformation and disorientation in chordotonal organs, apparently without uncoupling the cilia from the ECM, and consequently to locomotion defects. Moreover, impaired chemotaxis in atk mutant larvae suggests that, based on ATK protein localization, the ECM is also crucial for the correct assembly of chemosensory receptors. In addition to defining a novel ECM component, our findings show the importance of ECM integrity for the proper morphogenesis of ciliated organs in different sensory modalities.
Keywords: LRR proteins; chordotonal organ; dendritic cap; extracellular matrix; sensory cilium.
Figures




References
-
- Avidor-Reiss T., Maer A. M., Koundakjian E., Polyanovsky A., Keil T., et al. , 2004. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell 117: 527–539. - PubMed
-
- Barolo S., Walker R. G., Polyanovsky A. D., Freschi G., Keil T., et al. , 2000. A notch-independent activity of suppressor of hairless is required for normal mechanoreceptor physiology. Cell 103: 957–969. - PubMed
-
- Bodmer R., Carretto R., Jan Y. N., 1989. Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron 3: 21–32. - PubMed
-
- Brewster R., Bodmer R., 1995. Origin and specification of type II sensory neurons in Drosophila. Development 121: 2923–2936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

