Angiographic validation of the American College of Cardiology Foundation-the Society of Thoracic Surgeons Collaboration on the Comparative Effectiveness of Revascularization Strategies study
- PMID: 24496239
- PMCID: PMC5656245
- DOI: 10.1161/CIRCINTERVENTIONS.113.000679
Angiographic validation of the American College of Cardiology Foundation-the Society of Thoracic Surgeons Collaboration on the Comparative Effectiveness of Revascularization Strategies study
Abstract
Background: The goal of this study was to compare angiographic interpretation of coronary arteriograms by sites in community practice versus those made by a centralized angiographic core laboratory.
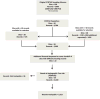
Methods and results: The study population consisted of 2013 American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR) records with 2- and 3- vessel coronary disease from 54 sites in 2004 to 2007. The primary analysis compared Registry (NCDR)-defined 2- and 3-vessel disease versus those from an angiographic core laboratory analysis. Vessel-level kappa coefficients suggested moderate agreement between NCDR and core laboratory analysis, ranging from kappa=0.39 (95% confidence intervals, 0.32-0.45) for the left anterior descending artery to kappa=0.59 (95% confidence intervals, 0.55-0.64) for the right coronary artery. Overall, 6.3% (n=127 out of 2013) of those patients identified with multivessel disease at NCDR sites had had 0- or 1-vessel disease by core laboratory reading. There was no directional bias with regard to overcall, that is, 12.3% of cases read as 3-vessel disease by the sites were read as <3-vessel disease by the core laboratory, and 13.9% of core laboratory 3-vessel cases were read as <3-vessel by the sites. For a subset of patients with left main coronary disease, registry overcall was not linked to increased rates of mortality or myocardial infarction.
Conclusions: There was only modest agreement between angiographic readings in clinical practice and those from an independent core laboratory. Further study will be needed because the implications for patient management are uncertain.
Keywords: angiography; catheterization; percutaneous coronary intervention; program evaluation.
Conflict of interest statement
The primary author reports no conflicts. Dr Peterson has received consulting fees and honoraria from Janssen Pharmaceuticals, Inc, Genentech, Boehringer Ingelheim as well as research grants from Eli Lilly and Janssen Pharmaceuticals, Inc. Dr Weintraub has received consulting fees and honoraria from Eli Lilly, Bristol Myers Squibb, Pfizer, and Amarin. Dr Gibson has received consulting fees and honoraria from Johnson & Johnson Corp, Bayer Corporation, Ischemix, Inc, BCRI, Sanofi-Aventis Corp, Genentech, Inc, Merck & Co, CSL Behring, Biogen Idec, Daiichi Sankyo, Inc, Bristol Meyer Squibb, The Medicines Company, Janssen Pharmaceuticals, Inc, Regado Biosciences, Inc, St. Jude Medical Corp., Ortho McNeil, Portola Pharmaceuticals, Eli Lilly, and GlaxoSmithKline, as well as research grants from Lantheus Medical Imaging, Janssen Pharmaceuticals, Inc, Bayer Corporation, Genentech Inc, Merck & Co, Atrium Medical Systems, Roche Diagnostics, Ikaria, Inc, Portola Pharmaceuticals, Johnson & Johnson Corp, Angel Medical Corporation, Volcano Corp, Stealth Peptides, Sanofi-Aventis, Walk Vascular, and St Jude’s Medical, as well as other financial support from UpToDate in Cardiovascular Medicine. All other authors report no conflicts.
Figures

References
-
- Costa RA, Reiber JH. QCA editorial. Int J Cardiovasc Imaging. 2011;27:155–156. - PubMed
-
- Brener SJ, Haq SA, Bose S, Sacchi TJ. Three-year survival after percutaneous coronary intervention according to appropriateness criteria for revascularization. J Invasive Cardiol. 2009;21:554–557. - PubMed
-
- Stone GW, Moses JW. Interventional cardiology: how should the appropriateness of PCI be judged? Nat Rev Cardiol. 2011;8:544–546. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

