Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy
- PMID: 24496315
- PMCID: PMC4016792
- DOI: 10.1038/mi.2013.123
Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy
Abstract
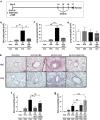
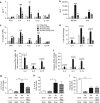
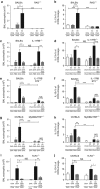
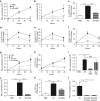
Helminth parasites such as the nematode Heligmosomoides polygyrus strongly inhibit T helper type 2 (Th2) allergy, as well as colitis and autoimmunity. Here, we show that the soluble excretory/secretory products of H. polygyrus (HES) potently suppress inflammation induced by allergens from the common fungus Alternaria alternata. Alternaria extract, when administered to mice intranasally with ovalbumin (OVA) protein, induces a rapid (1-48 h) innate response while also priming an OVA-specific Th2 response that can be evoked 14 days later by intranasal administration of OVA alone. In this model, HES coadministration with Alternaria/OVA suppressed early IL-33 release, innate lymphoid cell (ILC) production of IL-4, IL-5, and IL-13, and localized eosinophilia. Upon OVA challenge, type 2 ILC (ILC2)/Th2 cytokine production and eosinophilia were diminished in HES-treated mice. HES administration 6 h before Alternaria blocked the allergic response, and its suppressive activity was abolished by heat treatment. Administration of recombinant IL-33 at sensitization with Alternaria/OVA/HES abrogated HES suppression of OVA-specific responses at challenge, indicating that suppression of early Alternaria-induced IL-33 release could be central to the anti-allergic effects of HES. Thus, this helminth parasite targets IL-33 production as part of its armory of suppressive effects, forestalling the development of the type 2 immune response to infection and allergic sensitization.
Figures

References
-
- Eder W., Ege M.J., von Mutius E. The asthma epidemic. N. Engl. J. Med. 2006;355:2226–2235. - PubMed
-
- Yazdanbakhsh M., Kremsner P.G., van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. - PubMed
-
- Fleming J., Fabry Z. The hygiene hypothesis and multiple sclerosis. Ann. Neurol. 2007;61:85–89. - PubMed
-
- Fanta C.H. Asthma. N. Engl. J. Med. 2009;360:1002–1014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

