Essential role of program death 1-ligand 1 in regulatory T-cell-afforded protection against blood-brain barrier damage after stroke
- PMID: 24496394
- PMCID: PMC3939692
- DOI: 10.1161/STROKEAHA.113.004100
Essential role of program death 1-ligand 1 in regulatory T-cell-afforded protection against blood-brain barrier damage after stroke
Abstract
Background and purpose: Our recent research revealed that adoptively transferred regulatory T cells (Tregs) reduced acute ischemic brain injury by inhibiting neutrophil-derived matrix metalloproteinase-9 (MMP-9) and protecting against blood-brain barrier damage. The mechanisms underlying Treg interactions with neutrophils remain elusive. This study evaluates the contribution of program death 1-ligand 1 (PD-L1) to Treg-mediated neutrophil inhibition and neuroprotection after cerebral ischemia.
Methods: In vitro experiments were performed using a transwell system or a coculture system allowing cell-to-cell contact. Focal cerebral ischemia was induced in mice for 60 minutes. Tregs (2×10(6)) isolated from donor animals (wild-type or PD-L1-/-) were intravenously injected into ischemic recipients 2 hours after middle cerebral artery occlusion (MCAO). MMP-9 production, blood-brain barrier permeability, and brain infarct were assessed at 1 or 3 days after MCAO.
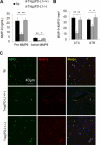
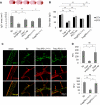
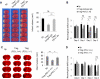
Results: In vitro experiments reveal that Treg-mediated inhibition of neutrophil MMP-9 required direct cell-to-cell contact. The suppression of MMP-9 was abolished when Tregs were pretreated with PD-L1 neutralizing antibodies or when neutrophils were pretreated with PD-1 antibodies. In vivo studies confirmed that intravenous administration of Tregs pretreated with PD-L1 antibodies or Tregs isolated from PD-L1-deficient mice failed to inhibit MMP-9 production by blood neutrophils 1 day after 60 minutes MCAO. Furthermore, the blood-brain barrier damage after MCAO was greatly ameliorated in PD-L1-competent Treg-treated mice but not in PD-L1-compromised Treg-treated mice. Consequently, PD-L1 dysfunction abolished Treg-mediated brain protection and neurological improvements 3 days after MCAO.
Conclusions: PD-L1 plays an essential role in the neuroprotection afforded by Tregs against cerebral ischemia by mediating the suppressive effect of Tregs on neutrophil-derived MMP-9.
Keywords: matrix metalloproteinase 9; neutrophils; programmed cell death 1 ligand 1; regulatory T-cells; stroke.
Figures



References
-
- Rosell A, Ortega-Aznar A, Alvarez-Sabin J, Fernandez-Cadenas I, Ribo M, Molina CA, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke; a journal of cerebral circulation. 2006;37:1399–1406. - PubMed
-
- Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. Mmp-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type iv collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

