How to make an intestine
- PMID: 24496613
- PMCID: PMC3912826
- DOI: 10.1242/dev.097386
How to make an intestine
Abstract
With the high prevalence of gastrointestinal disorders, there is great interest in establishing in vitro models of human intestinal disease and in developing drug-screening platforms that more accurately represent the complex physiology of the intestine. We will review how recent advances in developmental and stem cell biology have made it possible to generate complex, three-dimensional, human intestinal tissues in vitro through directed differentiation of human pluripotent stem cells. These are currently being used to study human development, genetic forms of disease, intestinal pathogens, metabolic disease and cancer.
Keywords: Directed differentiation; Embryonic stem cells; Gastrointestinal disease; Gut tube; Intestinal morphogenesis; Pluripotent stem cells.
Figures
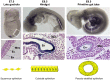
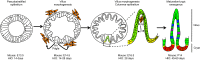
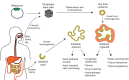
Similar articles
-
Generating human intestinal tissues from pluripotent stem cells to study development and disease.EMBO J. 2015 May 5;34(9):1149-63. doi: 10.15252/embj.201490686. Epub 2015 Mar 19. EMBO J. 2015. PMID: 25792515 Free PMC article. Review.
-
[From human pluripotent stem cells to custom-made intestinal organoids].Med Sci (Paris). 2019 Jun-Jul;35(6-7):549-555. doi: 10.1051/medsci/2019096. Epub 2019 Jul 5. Med Sci (Paris). 2019. PMID: 31274085 Review. French.
-
Organogenesis in a dish: modeling development and disease using organoid technologies.Science. 2014 Jul 18;345(6194):1247125. doi: 10.1126/science.1247125. Epub 2014 Jul 17. Science. 2014. PMID: 25035496 Review.
-
Generation and assembly of human brain region-specific three-dimensional cultures.Nat Protoc. 2018 Sep;13(9):2062-2085. doi: 10.1038/s41596-018-0032-7. Nat Protoc. 2018. PMID: 30202107 Free PMC article.
-
"Miniguts" from plucked human hair meet Crohn's disease.Z Gastroenterol. 2016 Aug;54(8):748-59. doi: 10.1055/s-0042-105520. Epub 2016 Jul 14. Z Gastroenterol. 2016. PMID: 27415403 English.
Cited by
-
Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age.EFSA J. 2017 May 31;15(5):e04849. doi: 10.2903/j.efsa.2017.4849. eCollection 2017 May. EFSA J. 2017. PMID: 32625502 Free PMC article.
-
Human Microphysiological Models of Intestinal Tissue and Gut Microbiome.Front Bioeng Biotechnol. 2020 Jul 31;8:725. doi: 10.3389/fbioe.2020.00725. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32850690 Free PMC article. Review.
-
Biologically inspired approaches to enhance human organoid complexity.Development. 2019 Apr 16;146(8):dev166173. doi: 10.1242/dev.166173. Development. 2019. PMID: 30992275 Free PMC article. Review.
-
Potential Application of Intestinal Organoids in Intestinal Diseases.Stem Cell Rev Rep. 2024 Jan;20(1):124-137. doi: 10.1007/s12015-023-10651-w. Epub 2023 Nov 8. Stem Cell Rev Rep. 2024. PMID: 37938407 Review.
-
Organoids recapitulate organs?Stem Cell Investig. 2018 Jan 17;5:3. doi: 10.21037/sci.2018.01.02. eCollection 2018. Stem Cell Investig. 2018. PMID: 29430459 Free PMC article. No abstract available.
References
-
- Ameri J., Ståhlberg A., Pedersen J., Johansson J. K., Johannesson M. M., Artner I., Semb H. (2009). FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells 28, 45–56 - PubMed
-
- Batts L. E., Polk D. B., Dubois R. N., Kulessa H. (2006). Bmp signaling is required for intestinal growth and morphogenesis. Dev. Dyn. 235, 1563–1570 - PubMed
-
- Burns A. J., Delalande J. M., Le Douarin N. M. (2002). In ovo transplantation of enteric nervous system precursors from vagal to sacral neural crest results in extensive hindgut colonisation. Development 129, 2785–2796 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

