Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo
- PMID: 24496619
- PMCID: PMC3912828
- DOI: 10.1242/dev.103036
Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo
Erratum in
- Development. 2014 Apr;141(7):1599. Rubel, Edwin W [added]
Abstract
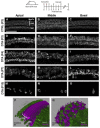
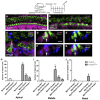
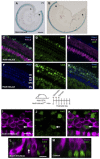
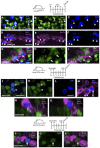
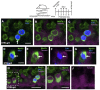
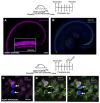
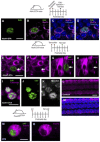
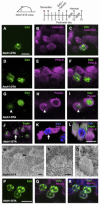
Loss of cochlear hair cells in mammals is currently believed to be permanent, resulting in hearing impairment that affects more than 10% of the population. Here, we developed two genetic strategies to ablate neonatal mouse cochlear hair cells in vivo. Both Pou4f3(DTR/+) and Atoh1-CreER™; ROSA26(DTA/+) alleles allowed selective and inducible hair cell ablation. After hair cell loss was induced at birth, we observed spontaneous regeneration of hair cells. Fate-mapping experiments demonstrated that neighboring supporting cells acquired a hair cell fate, which increased in a basal to apical gradient, averaging over 120 regenerated hair cells per cochlea. The normally mitotically quiescent supporting cells proliferated after hair cell ablation. Concurrent fate mapping and labeling with mitotic tracers showed that regenerated hair cells were derived by both mitotic regeneration and direct transdifferentiation. Over time, regenerated hair cells followed a similar pattern of maturation to normal hair cell development, including the expression of prestin, a terminal differentiation marker of outer hair cells, although many new hair cells eventually died. Hair cell regeneration did not occur when ablation was induced at one week of age. Our findings demonstrate that the neonatal mouse cochlea is capable of spontaneous hair cell regeneration after damage in vivo. Thus, future studies on the neonatal cochlea might shed light on the competence of supporting cells to regenerate hair cells and on the factors that promote the survival of newly regenerated hair cells.
Keywords: Atoh1; Diphtheria toxin; Direct transdifferentiation; Fate mapping; Lgr5; Mitotic regeneration.
Figures


Similar articles
-
In Vivo Cochlear Hair Cell Generation and Survival by Coactivation of β-Catenin and Atoh1.J Neurosci. 2015 Jul 29;35(30):10786-98. doi: 10.1523/JNEUROSCI.0967-15.2015. J Neurosci. 2015. PMID: 26224861 Free PMC article.
-
Multiple supporting cell subtypes are capable of spontaneous hair cell regeneration in the neonatal mouse cochlea.Development. 2019 Feb 15;146(4):dev171009. doi: 10.1242/dev.171009. Development. 2019. PMID: 30770379 Free PMC article.
-
Conditional Overexpression of Net1 Enhances the Trans-Differentiation of Lgr5+ Progenitors into Hair Cells in the Neonatal Mouse Cochlea.Cell Prolif. 2025 Apr;58(4):e13787. doi: 10.1111/cpr.13787. Epub 2024 Dec 15. Cell Prolif. 2025. PMID: 39675772 Free PMC article.
-
Atoh1 gene therapy in the cochlea for hair cell regeneration.Expert Opin Biol Ther. 2015 Mar;15(3):417-30. doi: 10.1517/14712598.2015.1009889. Epub 2015 Feb 3. Expert Opin Biol Ther. 2015. PMID: 25648190 Review.
-
Concise Review: Regeneration in Mammalian Cochlea Hair Cells: Help from Supporting Cells Transdifferentiation.Stem Cells. 2017 Mar;35(3):551-556. doi: 10.1002/stem.2554. Epub 2017 Jan 19. Stem Cells. 2017. PMID: 28102558 Review.
Cited by
-
Taurine enhances excitability of mouse cochlear neural stem cells by selectively promoting differentiation of glutamatergic neurons over GABAergic neurons.Neurochem Res. 2015 May;40(5):924-31. doi: 10.1007/s11064-015-1546-9. Epub 2015 Mar 1. Neurochem Res. 2015. PMID: 25725997
-
The quest for restoring hearing: Understanding ear development more completely.Bioessays. 2015 Sep;37(9):1016-27. doi: 10.1002/bies.201500044. Epub 2015 Jul 24. Bioessays. 2015. PMID: 26208302 Free PMC article. Review.
-
A Neurophysiological Study of Musical Pitch Identification in Mandarin-Speaking Cochlear Implant Users.Neural Plast. 2020 Jul 22;2020:4576729. doi: 10.1155/2020/4576729. eCollection 2020. Neural Plast. 2020. PMID: 32774355 Free PMC article.
-
Evaluation of Nestin Expression in the Developing and Adult Mouse Inner Ear.Stem Cells Dev. 2016 Oct 1;25(19):1419-32. doi: 10.1089/scd.2016.0176. Epub 2016 Sep 7. Stem Cells Dev. 2016. PMID: 27474107 Free PMC article.
-
Inhibition of H3K9me2 Reduces Hair Cell Regeneration after Hair Cell Loss in the Zebrafish Lateral Line by Down-Regulating the Wnt and Fgf Signaling Pathways.Front Mol Neurosci. 2016 May 26;9:39. doi: 10.3389/fnmol.2016.00039. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27303264 Free PMC article.
References
-
- Abrahamsen B., Zhao J., Asante C. O., Cendan C. M., Marsh S., Martinez-Barbera J. P., Nassar M. A., Dickenson A. H., Wood J. N. (2008). The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321, 702–705 - PubMed
-
- Adler H. J., Raphael Y. (1996). New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci. Lett. 205, 17–20 - PubMed
-
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. (2002). Molecular Biology of the Cell. New York, NJ: Garland Science;
-
- Baird R. A., Steyger P. S., Schuff N. R. (1996). Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann. N. Y. Acad. Sci. 781, 59–70 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DC006471/DC/NIDCD NIH HHS/United States
- DC011043/DC/NIDCD NIH HHS/United States
- R21 DC008800/DC/NIDCD NIH HHS/United States
- K08 DC011043/DC/NIDCD NIH HHS/United States
- DC009393/DC/NIDCD NIH HHS/United States
- P30DC010363/DC/NIDCD NIH HHS/United States
- F31 DC009393/DC/NIDCD NIH HHS/United States
- P30 DC010363/DC/NIDCD NIH HHS/United States
- CA21765/CA/NCI NIH HHS/United States
- DC006471/DC/NIDCD NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- DC010310/DC/NIDCD NIH HHS/United States
- F32 DC010310/DC/NIDCD NIH HHS/United States
- DC008800/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

