Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90
- PMID: 24496637
- PMCID: PMC4038785
- DOI: 10.1093/toxsci/kfu022
Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90
Erratum in
-
Toxicity Evaluation of Bisphenol A Administered by Gavage to Sprague Dawley Rats From Gestation Day 6 Through Postnatal Day 90.Toxicol Sci. 2016 Sep;153(1):212. doi: 10.1093/toxsci/kfw123. Epub 2016 Aug 9. Toxicol Sci. 2016. PMID: 27506224 Free PMC article. No abstract available.
Abstract
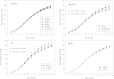
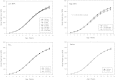
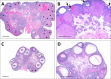
Bisphenol A (BPA) is a high production volume industrial chemical to which there is widespread human oral exposure. Guideline studies used to set regulatory limits detected adverse effects only at doses well above human exposures and established a no-observed-adverse-effect level (NOAEL) of 5 mg/kg body weight (bw)/day. However, many reported animal studies link BPA to potentially adverse effects on multiple organ systems at doses below the NOAEL. The primary goals of the subchronic study reported here were to identify adverse effects induced by orally (gavage) administered BPA below the NOAEL, to characterize the dose response for such effects and to determine doses for a subsequent chronic study. Sprague Dawley rat dams were dosed daily from gestation day 6 until the start of labor, and their pups were directly dosed from day 1 after birth to termination. The primary focus was on seven equally spaced BPA doses (2.5-2700 μg/kg bw/day). Also included were a naïve control, two doses of ethinyl estradiol (EE2) to demonstrate the estrogen responsiveness of the animal model, and two high BPA doses (100,000 and 300,000 μg/kg bw/day) expected from guideline studies to produce adverse effects. Clear adverse effects of BPA, including depressed gestational and postnatal body weight gain, effects on the ovary (increased cystic follicles, depleted corpora lutea, and antral follicles), and serum hormones (increased serum estradiol and prolactin and decreased progesterone), were observed only at the two high doses of BPA. BPA-induced effects partially overlapped those induced by EE2, consistent with the known weak estrogenic activity of BPA.
Keywords: 90-day study; Bisphenol A; Sprague Dawley rat; ethinyl estradiol.
Figures
Comment in
-
Response to Hunt et al., invalid controls undermine conclusions of FDA studies.Toxicol Sci. 2014 Sep;141(1):3. doi: 10.1093/toxsci/kfu102. Toxicol Sci. 2014. PMID: 24863966 Free PMC article. No abstract available.
-
Invalid controls undermine conclusions of FDA studies.Toxicol Sci. 2014 Sep;141(1):1-2. doi: 10.1093/toxsci/kfu179. Toxicol Sci. 2014. PMID: 25232149 Free PMC article.
Similar articles
-
Investigation of the effects of subchronic low dose oral exposure to bisphenol A (BPA) and ethinyl estradiol (EE) on estrogen receptor expression in the juvenile and adult female rat hypothalamus.Toxicol Sci. 2014 Jul;140(1):190-203. doi: 10.1093/toxsci/kfu074. Epub 2014 Apr 20. Toxicol Sci. 2014. PMID: 24752507 Free PMC article.
-
Effects of oral exposure to bisphenol A on gene expression and global genomic DNA methylation in the prostate, female mammary gland, and uterus of NCTR Sprague-Dawley rats.Food Chem Toxicol. 2015 Jul;81:92-103. doi: 10.1016/j.fct.2015.04.009. Epub 2015 Apr 8. Food Chem Toxicol. 2015. PMID: 25862956 Free PMC article.
-
Comparison of life-stage-dependent internal dosimetry for bisphenol A, ethinyl estradiol, a reference estrogen, and endogenous estradiol to test an estrogenic mode of action in Sprague Dawley rats.Toxicol Sci. 2014 May;139(1):4-20. doi: 10.1093/toxsci/kfu021. Epub 2014 Feb 4. Toxicol Sci. 2014. PMID: 24496641 Free PMC article.
-
CLARITY-BPA academic laboratory studies identify consistent low-dose Bisphenol A effects on multiple organ systems.Basic Clin Pharmacol Toxicol. 2019 Aug;125 Suppl 3(Suppl 3):14-31. doi: 10.1111/bcpt.13125. Epub 2018 Oct 17. Basic Clin Pharmacol Toxicol. 2019. PMID: 30207065 Free PMC article. Review.
-
Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A.Crit Rev Toxicol. 2011 Apr;41(4):263-91. doi: 10.3109/10408444.2011.558487. Crit Rev Toxicol. 2011. PMID: 21438738 Free PMC article. Review.
Cited by
-
Invalid controls undermine conclusions of FDA studies.Toxicol Sci. 2014 Sep;141(1):1-2. doi: 10.1093/toxsci/kfu179. Toxicol Sci. 2014. PMID: 25232149 Free PMC article.
-
Achieving CLARITY on bisphenol A, brain and behaviour.J Neuroendocrinol. 2020 Jan;32(1):e12730. doi: 10.1111/jne.12730. Epub 2019 May 26. J Neuroendocrinol. 2020. PMID: 31063678 Free PMC article. Review.
-
The Conflict between Regulatory Agencies over the 20,000-Fold Lowering of the Tolerable Daily Intake (TDI) for Bisphenol A (BPA) by the European Food Safety Authority (EFSA).Environ Health Perspect. 2024 Apr;132(4):45001. doi: 10.1289/EHP13812. Epub 2024 Apr 9. Environ Health Perspect. 2024. PMID: 38592230 Free PMC article.
-
Evidence for bisphenol A-induced female infertility: a review (2007-2016).Fertil Steril. 2016 Sep 15;106(4):827-56. doi: 10.1016/j.fertnstert.2016.06.027. Epub 2016 Jul 12. Fertil Steril. 2016. PMID: 27417731 Free PMC article. Review.
-
On the Use and Interpretation of Areola/Nipple Retention as a Biomarker for Anti-androgenic Effects in Rat Toxicity Studies.Front Toxicol. 2021 Oct 27;3:730752. doi: 10.3389/ftox.2021.730752. eCollection 2021. Front Toxicol. 2021. PMID: 35295101 Free PMC article. Review.
References
-
- Andrews P., Freyberger A., Hartmann E., Eiben R., Loof I., Schmidt U., Temerowski M., Folkerts A., Stahl B., Kayser M. Sensitive detection of the endocrine effects of the estrogen analogue ethinylestradiol using a modified enhanced subacute rat study protocol (OECD Test Guideline no. 407) Arch. Toxicol. 2002;76:194–202. - PubMed
-
- Barraclough C. A. Production of anovulatory, sterile rats by single injections of testosterone propionate. Endocrinology. 1961;68:62–67. - PubMed
-
- Bell D. D., Zucker I. Sex differences in body weight and eating: Organization and activation by gonadal hormones in the rat. Physiol. Behav. 1971;7:27–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

