Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model
- PMID: 24496748
- PMCID: PMC4209016
- DOI: 10.1002/stem.1654
Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model
Abstract
Mesenchymal stem cells (MSCs) possess unique paracrine and immunosuppressive properties, which make them useful candidates for cellular therapy. Here, we address how cellular senescence influences the therapeutic potential of human MSCs (hMSCs). Senescence was induced in bone marrow-derived hMSC cultures with gamma irradiation. Control and senescent cells were tested for their immunoregulatory activity in vitro and in vivo, and an extensive molecular characterization of the phenotypic changes induced by senescence was performed. We also compared the gene expression profiles of senescent hMSCs with a collection of hMSCs used in an ongoing clinical study of Graft Versus Host disease (GVHD). Our results show that senescence induces extensive phenotypic changes in hMSCs and abrogates their protective activity in a murine model of LPS-induced lethal endotoxemia. Although senescent hMSCs retain an ability to regulate the inflammatory response on macrophages in vitro, and, in part retain their capacity to significantly inhibit lymphocyte proliferation, they have a severely impaired migratory capacity in response to proinflammatory signals, which is associated with an inhibition of the AP-1 pathway. Additionally, expression analysis identified PLEC, C8orf48, TRPC4, and ZNF14, as differentially regulated genes in senescent hMSCs that were similarly regulated in those hMSCs which failed to produce a therapeutic effect in a GVHD trial. All the observed phenotypic alterations were confirmed in replicative-senescent hMSCs. In conclusion, this study highlights important changes in the immunomodulatory phenotype of senescent hMSCs and provides candidate gene signatures which may be useful to evaluate the therapeutic potential of hMSCs used in future clinical studies.
Keywords: Cellular therapy; Immunotherapy; Mesenchymal stem cells; Senescence.
© 2014 AlphaMed Press.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures
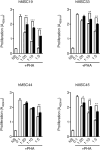




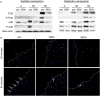
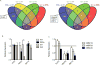
References
-
- Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009;1176:101–117. - PubMed
-
- Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128–134. - PubMed
-
- Stagg J, Pommey S, Eliopoulos N, et al. Interferon-gamma-stimulated marrow stromal cells: A new type of nonhematopoietic antigen-presenting cell. Blood. 2006;107:2570–2577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

