Iron-regulated expression of alginate production, mucoid phenotype, and biofilm formation by Pseudomonas aeruginosa
- PMID: 24496793
- PMCID: PMC3950519
- DOI: 10.1128/mBio.01010-13
Iron-regulated expression of alginate production, mucoid phenotype, and biofilm formation by Pseudomonas aeruginosa
Abstract
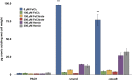
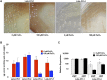
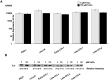
Pseudomonas aeruginosa strains of non-cystic fibrosis (non-CF) origin do not produce significant amounts of extracellular alginate and are nonmucoid. In CF, such isolates can become mucoid through mutation of one of the genes (mucA, mucB, mucC, or mucD) that produce regulatory factors that sequester AlgU, required for increased expression of alginate genes. Mutation of the muc genes in the nonmucoid PAO1, PA14, PAKS-1, and Ps388 strains led to increased levels of extracellular alginate and an obvious mucoid phenotype, but only under iron-limiting growth conditions (≤5 µM), not under iron-replete conditions (≥10 µM). In contrast, >50% of P. aeruginosa isolates from chronic CF pulmonary infections expressed increased levels of alginate and mucoidy both under iron-limiting and iron-replete conditions (i.e., iron-constitutive phenotype). No single iron regulatory factor (e.g., Fur, PvdS) was associated with this loss of iron-regulated alginate expression and mucoidy in these CF isolates. However, the loss of only pyoverdine production, or its uptake, abrogated the ability of P. aeruginosa to produce a robust biofilm that represents the Psl-type of biofilm. In contrast, we show that mutation of the pyoverdine and pyochelin biosynthesis genes and the pyoverdine receptor (FpvA) lead to iron-constitutive expression of the key alginate biosynthesis gene, algD, and an explicitly mucoid phenotype in both iron-limiting and iron-replete conditions. These data indicate that alginate production and mucoidy, in contrast to other types of biofilms produced by P. aeruginosa, are substantially enhanced under iron limitation. These results also have compelling implications in relation to the use of iron chelators in the treatment of P. aeruginosa CF infections.
Importance: Pseudomonas aeruginosa is a leading model for the investigation of biofilms. While data have been generated about the role of iron in alginate-independent (Psl/Pel) biofilm development, there is a paucity of data regarding the role of iron in alginate production and its associated mucoid phenotype. We demonstrate that biologically relevant levels of iron that exist in the airway mucus of cystic fibrosis (CF) patients have a substantial influence on production of alginate and the overt mucoid phenotype, pathognomonic of P. aeruginosa infections in CF. Mucoid mutants of non-CF P. aeruginosa isolates are mucoid only under iron limitation and do not express increased levels of alginate under iron-replete growth conditions. However, a significant number of long-term CF isolates lost their iron-regulated expression of increased alginate production and mucoidy and became iron constitutive for these properties. In contrast to the formation of Psl-type biofilms, increasing iron limitation ultimately leads to an iron-constitutive expression of alginate and mucoidy.
Figures



Similar articles
-
Pseudomonas aeruginosa in cystic fibrosis: role of mucC in the regulation of alginate production and stress sensitivity.Microbiology (Reading). 1997 Nov;143 ( Pt 11):3473-3480. doi: 10.1099/00221287-143-11-3473. Microbiology (Reading). 1997. PMID: 9387225
-
Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients.Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8377-81. doi: 10.1073/pnas.90.18.8377. Proc Natl Acad Sci U S A. 1993. PMID: 8378309 Free PMC article.
-
Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung.Microbiology (Reading). 1999 Jun;145 ( Pt 6):1349-1357. doi: 10.1099/13500872-145-6-1349. Microbiology (Reading). 1999. PMID: 10411261
-
Conversion to mucoidy in Pseudomonas aeruginosa.Biotechnology (N Y). 1993 Oct;11(10):1133-6. doi: 10.1038/nbt1093-1133. Biotechnology (N Y). 1993. PMID: 7764093 Review.
-
The algD promoter: regulation of alginate production by Pseudomonas aeruginosa in cystic fibrosis.Cell Mol Biol Res. 1993;39(4):371-6. Cell Mol Biol Res. 1993. PMID: 8312973 Review.
Cited by
-
High-quality genome-scale metabolic modelling of Pseudomonas putida highlights its broad metabolic capabilities.Environ Microbiol. 2020 Jan;22(1):255-269. doi: 10.1111/1462-2920.14843. Epub 2019 Nov 11. Environ Microbiol. 2020. PMID: 31657101 Free PMC article.
-
Extracellular haem utilization by the opportunistic pathogen Pseudomonas aeruginosa and its role in virulence and pathogenesis.Adv Microb Physiol. 2021;79:89-132. doi: 10.1016/bs.ampbs.2021.07.004. Epub 2021 Aug 13. Adv Microb Physiol. 2021. PMID: 34836613 Free PMC article.
-
Static Growth Promotes PrrF and 2-Alkyl-4(1H)-Quinolone Regulation of Type VI Secretion Protein Expression in Pseudomonas aeruginosa.J Bacteriol. 2020 Nov 19;202(24):e00416-20. doi: 10.1128/JB.00416-20. Print 2020 Nov 19. J Bacteriol. 2020. PMID: 33020221 Free PMC article.
-
Anti-Pathogenic Functions of Non-Digestible Oligosaccharides In Vitro.Nutrients. 2020 Jun 16;12(6):1789. doi: 10.3390/nu12061789. Nutrients. 2020. PMID: 32560186 Free PMC article. Review.
-
Formation of Pseudomonas aeruginosa Biofilms in Full-thickness Scald Burn Wounds in Rats.Sci Rep. 2019 Sep 20;9(1):13627. doi: 10.1038/s41598-019-50003-8. Sci Rep. 2019. PMID: 31541159 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

